Internal Medicine II
Research Focus
General Facts
Research
Selected Publications
Selected Funding, Collaboration
Keywords: Clinical and experimental infectious diseases, clinical immunology, rheumatology, experimental and clinical pneumology, host-pathogen interaction, innate immunity, anaemia of inflammation, lipid metabolism, diagnostic biomarkers, iron homeostasis
Research (ÖSTAT Classification) : 302030, 301902, 302072, 303031
Research Focus
Our research focuses on elucidation of the basic principles and clinical management of diseases at the bench and at the bedside, in order gain a detailed understanding of the biochemical, molecular and translational aspects of diseases, seen within the spectrum of our clinical expertise in internal medicine, focusing on infectious diseases, immunology, pneumology, rheumatology and general internal medicine.
General Facts
The department focuses on all clinical and scientific aspects of infectious diseases (ID), clinical immunology, pneumology and rheumatology.
On the clinical side, the department is a referral centre with special in-patient and outpatient facilities for ID, pneumology and rheumatology and it is home to a diagnostic laboratory for ID and rheumatology. We also operate facilities for ultrasound, echocardiography, bronchoscopy and pulmonary function testing. Moreover, we cover all general aspects of internal medicine and we appreciate its interconnections with all related medical disciplines.
On the scientific side, several branches of research flourish under the umbrella of the department. Our research groups have a strong background in cellular and molecular biology as well as immunology, using up-to-date analytical techniques, and we have elucidated numerous mechanisms, diagnostic algorithms and novel therapeutic principles in a variety of diseases, including anaemia of inflammation, atherosclerosis, iron dyshomeostasis, infections with extracellular and intracellular microbes, host-pathogen interplay, and the interconnections of iron, energy and lipid metabolism with the immune system. In our research laboratories and on our clinical wards, we investigate relevant topics in clinical ID, rheumatology and pneumology, using a wide range of up-to-date in silico, in vitro and in vivo technologies, including investigations into new and emerging diseases and the use of biomarkers. Our laboratories are well equipped with modern infrastructure, such as for multiplex quantitative PCR, multicolour flow cytometry, confocal microscopy and in vivo chemiluminescence imaging.
Research
The overarching aim of our research groups is to translate molecular findings from clinical samples and disease models into better diagnostic and therapeutic tools, in order to improve the care of patients with infectious, inflammatory, metabolic, pulmonary and rheumatological disorders. Given the strong background of our research groups in iron homeostasis, lipid metabolism and translational medicine, the focus of our combined research efforts is on the complex interplay of iron and cholesterol handling in infectious, inflammatory and metabolic diseases, on the pathophysiology and therapy of anaemia of inflammation (AI), on the improvement of rheumatological disease classification, and on the clinical management of patients with pulmonary diseases.
Infectious Diseases – From Bacteria to Respiratory Viruses
Infectious agents are fought off by the concerted action of innate and acquired immune mechanisms. A central feature of the innate immune response to pathogens, including viruses, bacteria and fungi, is the sequestration of nutrients such as iron and lipids. A range of mechanisms has evolved to reduce the availability of iron and other micronutrients to pathogenic micro-organisms. Collectively, the mechanisms of nutrient withdrawal from microbes are termed “nutritional immunity”. Owing to its essential role for the growth and proliferation of microbial cells, iron is a major target of the nutritional immune response.
Disorders of iron homeostasis are both frequent and important to the clinical course and outcome of underlying and coexisting diseases. They are also relevant for global public health. For example, low-income countries have an especially high prevalence of iron deficiency anaemia, both in children and in adults. However, region-wide oral iron supplementation in these countries has resulted in higher rates of severe infection, for reasons that remain largely unknown. We are interested in the impact of iron supplementation on the immune system and on the course of bacterial infections. We have observed that iron supplementation fuels the proliferation of Salmonella Typhimurium in macrophages (Fig. 1), resulting in increased bacterial loads and poor clinical outcome for systemic infections. Our ongoing studies will have a major impact on preventive iron supplementation programmes in low-income countries and on iron fortification in food manufacture in high-income countries.
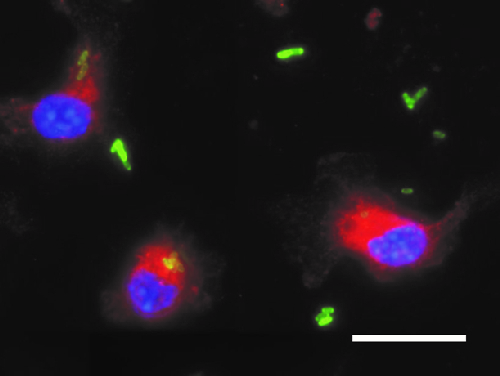
Fig. 1: Bone marrow-derived macrophages were loaded with iron and then infected with Salmonella Typhimurium expressing green fluorescent protein (GFP). Staining for lysosomal-associated membrane protein (LAMP)-1 was used to follow the intracellular trafficking of the bacteria along the phagolysosomal pathway. Some bacteria remain in the extracellular space. Blue: DAPI. Red: LAMP-1. Green: GFP Salmonella. Scale bar: 20 µm.
This diagram is provided courtesy of Manuel Grander.
We also want to understand how iron is compartmentalised in the host organism during infections and malignancies. Specifically, different mechanisms must exist to withhold iron from extracellular pathogens such as E. coli and Aspergillus fumigatus (or from malignant cells), as opposed to intracellular pathogens such as viruses, Chlamydia, Listeria or Salmonella species. The mechanisms involved need to be adapted and fine-tuned, depending on type and localisation of the noxious agent confronting the immune system. Delineating the signalling and metabolic pathways from pathogen or danger recognition to iron sequestration will allow us to characterise relevant mechanisms and to identify druggable targets for future therapeutic intervention.
In patients treated for sepsis, the most severe form of bacterial infection, we found that elevated ferritin in plasma, a biomarker both of iron storage and of inflammation, was associated with impaired survival. Specifically, in a prospective study of patients admitted to the medical intensive care unit (ICU) for sepsis, we found positive correlations of increased plasma iron and ferritin concentration upon ICU admission with the severity of organ failure (SOFA) score and with mortality. Collectively, our results and ongoing studies demonstrate that iron overload as a result of various causes leads to impaired immunity to microbes and to a severe course of infectious diseases.
We have investigated the epidemiology and multiple clinical aspects of respiratory viral and bacterial infections over many years, with a focus on influenza and pneumococcal/Leginonella pneumonia. The emergence of the COVID-19 pandemic also directed our focus towards the scientific aspects of this infection. We have described specific clinical presentations of COVID-19, the interaction of anaemia, disturbances of iron homeostasis and course of infection, we have identified biomarkers for disease prediction, and we have analysed the clinical utility of different COVID-19 diagnostic tests.
Iron, Immunity and Inflammatory Disorders
While many of the negative effects of iron on the course of infections are linked to iron storage in macrophages, surplus iron also impairs the function of lymphocytes. In mammary carcinoma, we found that dietary iron supplementation promoted tumour growth, because iron impaired the production of interleukin (IL)-2 and interferon (IFN)-γ, two major cytokine products of CD8+ cytotoxic T-cells (Fig. 2). Importantly, dietary iron supplementation also undermined the anti-tumour effects of immunotherapy and resulted in reduced response to treatment with a monoclonal antibody blocking the programmed cell death ligand (PD-L)-1, administered as a checkpoint inhibitor.
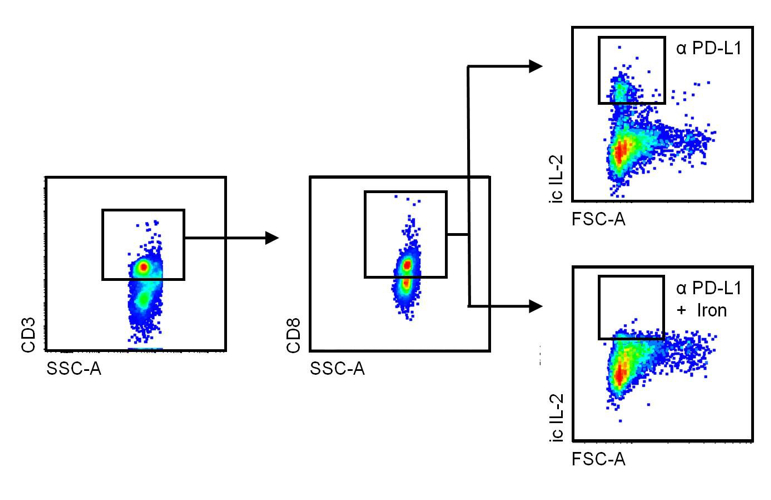
Fig. 2: Tumour-related leukocytes were isolated from mammary carcinoma IL-2 production by CD3+. CD8+ cytotoxic T-cells were assessed by means of intracellular staining, using multicolour flow cytometry. The dot plots show the gating strategy and the fact that iron inhibits IL-2 production by cytotoxic T-cells following treatment with a checkpoint-inhibiting antibody (α) that blocks programmed cell death ligand (PD-L)-1.
This diagram was modified from a version published by Piotr Tymoszuk and Christa Pfeifhofer-Obermair in Front. Oncol., 04 December 2020, https://doi.org/10.3389/fonc.2020.584477.
In our atherosclerosis and lipid metabolism research group, we elucidate the interplay of iron and cholesterol homeostasis with innate immunity in a translational manner. By combining human genetic data with functional experiments, we identified an unexpected connection between iron homeostasis and cholesterol transport: we found that a single nucleotide polymorphism (SNP) in the haemochromatosis gene HFE is associated with reduced levels of atherogenic low-density lipoprotein (LDL) cholesterol in human plasma. We saw that the underlying molecular mechanism is located in the liver, where HFE acts as a negative regulator of the LDL receptor. Kupffer cells act as gatekeepers of cholesterol homeostasis, as they accept LDL-derived cholesterol from plasma and transfer it to adjacent hepatocytes (Fig. 3) in a way that depends upon ATP-binding cassette transporter Abca1, which is regulated by iron disposal.
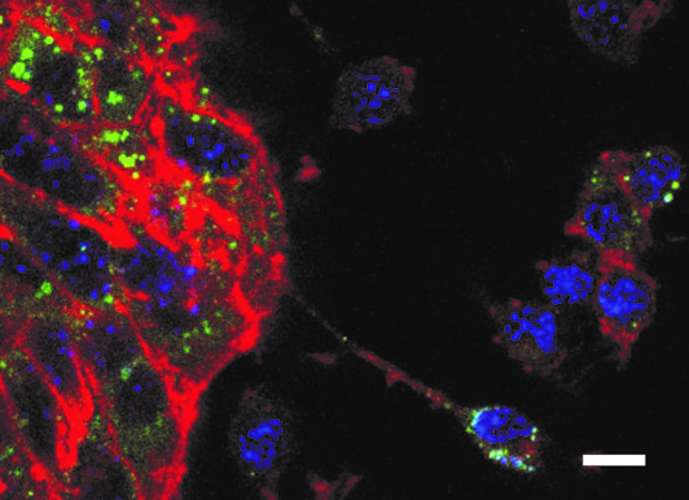
Fig. 3: Co-culture of Kupffer cells (small cells in the right section of the figure) and hepatocytes(conglomerate of large tubulin-rich cells in the left section of the figure). After loading of Kupffercells with cholesterol, the transfer of cholesterol to adjacent hepatocytes can be observed. Blue: DAPI.Red: tubulin. Green: cholesterol. Scale bar: 10 µm. This diagram is provided courtesy of Egon Demetz.
In conclusion, our research continues to reveal novel regulatory interactions between iron and lipid metabolism and immune cell biology. Our findings have important implications for existing therapies and identify promising targets for the treatment of inflammation-driven and metabolic disorders, including cancer, atherosclerosis and hypercholesterolaemia.
Rheumatological Diseases
Rheumatological diseases are among the most prevalent of inflammatory disorders. Medical scientists in the department are actively involved with improving diagnosis and treatment of these diseases, in national and international expert committees.
One of our aims is the international standardisation of autoantibody diagnosis, which is relevant for the classification of autoimmune and inflammatory disorders, including connective tissue diseases associated with antinuclear antibodies (ANA) and small-vessel vasculitides associated with antineutrophil cytoplasmic antibodies (ANCA). We work in close collaboration with the European Autoimmunity Standardisation Initiative (EASI) and with other members of the International Consensus on ANA Patterns (ICAP).
In large-vessel vasculitides and in arthritides, imaging is key to initial diagnostic work-up, disease classification and the identification of patients at increased risk of severe course of disease. Together with other national and international experts, the department participates in the compilation of evidence-based guidelines for the diagnosis and treatment of giant cell arteritis. In ongoing clinical studies, we are extending our expertise in ultrasound of the musculoskeletal and vascular systems to other rheumatological disorders, such as polymyalgia rheumatica, psoriatic arthritis and rheumatoid arthritis.
In addition to laboratory and ultrasound studies on rheumatological diseases, a major research focus of the department is on one of its most frequent clinical consequences, i.e. anaemia of inflammation (AI).
Anaemia of Inflammation
AI is a classic example of the connection between iron homeostasis and inflammation. Iron storage in macrophages is a major contributing factor in the pathogenesis of AI and renders iron unavailable for haemoglobin synthesis and red blood cell production. We have previously identified crucial pathways leading to AI mainly focusing on effects of cytokines such as interferon-gamma, interleukin-6 or interleukin-10 but also of bacterial lipopolysaccharides on iron homeostasis in macrophages and by elucidating complex regulatory interaction of iron homeostasis with central innate immune effector pathways. As an example hormonal and cytokine regulators, such as hepcidin and bone morphogenetic protein (BMP)-6, increase the iron content of macrophages and induce the iron storage protein ferritin. Not only are hepcidin and BMP6 therefore relevant for the diagnosis of AI; they are also potential targets for therapeutic intervention. Accordingly, we were recently able to demonstrate that a fully human anti-BMP6 antibody is safe and effective in the treatment of AI, because it inhibits hepcidin synthesis and restores the presence of the iron exporter ferroportin in red blood cells, thus promoting the maturation of their precursors in the bone marrow.
Together, our studies continue to identify novel links between inflammation and iron homeostasis, for a better understanding of pathophysiological mechanisms and the rational design of future pharmacological interventions.
Pulmonary Diseases Including Sequelae of COVID-19
The department runs long-term studies on pneumonia, chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH). For several months, the pandemic spread of severe acute respiratory syndrome coronavirus (SARS-CoV)-2 has posed an additional challenge to our physicians and to the whole Tyrolean healthcare system. We continue to face this challenge on the front line, both clinically and scientifically. In a major effort against coronavirus disease 2019 (COVID-19), the department, in close collaboration with the Hospital Pharmacy and the Central Institute of Clinical Laboratory Diagnostics, tested pharyngeal swab sets, virus transport media and rapid antigen tests, in order to validate their diagnostic performance.
In ongoing clinical trials, we are investigating the efficacy of specific treatments against SARS-CoV-2 and the genetic, immunological, hormonal and nutritional risk factors for severe disease courses, such as pneumonia and hyperinflammation syndrome as well as delayed cardiopulmonary recovery. We established the prospective “CovILD trial” for systematic cardiopulmonary follow-up of patients after COVID-19 and have already been able to report that elevated plasma ferritin levels and AI are associated with the severity of COVID-19. In COVID-19, there is therefore also a close connection between iron homeostasis and course of infection.
During the outbreak of COVID-19, we also experienced a dramatic decrease in hospitalisations due to COPD. This observation prompted us to use the data analysis tool “Google Trends” to investigate whether patients suffering from COPD and/or asthma may have consulted the internet for self-medication advice. We saw that patients diagnosed with asthma or COPD know alarmingly little about their disease and its treatment. On a global level, significant misinformation exists about the standard therapies. Our findings emphasise the fact that there is an urgent need for easier access to valid patient information and up-to-date treatment guidelines.
In the department, the medical scientists with a clinical focus on either pneumology or infectious diseases continue to make a significant contribution to improvement of the diagnosis and clinical management of COVID-19 patients (Fig. 4), whilst maintaining the highest standard of medical care for patients with other types of infectious or pulmonary diseases.
Since the 1960s, the Austrian Dialysis and Transplant Registry has collected individual data on all patients with end-stage renal disease who require dialysis or kidney transplantation. Since its move to Innsbruck in 2018, the registry has undergone transformation into web-based data repository, following rules set out in GDPR. The ÖDTR supports national healthcare authorities in the planning of regional dialysis facility resource allocation. In addition, it provides various quality-control programmes of the Austrian Society of Nephrology. Because of the high quality and completeness of the data collected, the registry is also an outstanding source for academic research. In addition to internal projects, the ÖDTR collaborates with national and international researchers and organisations (such as the European Renal Association – European Dialysis and Transplant Association) under the guidance of an external advisory board.
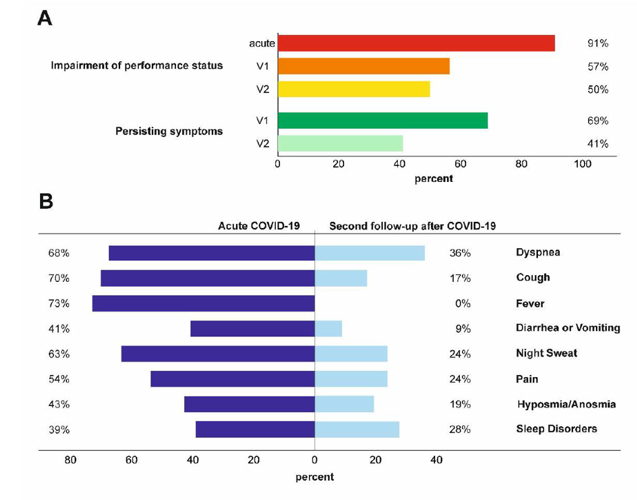
Fig. 4: Persisting symptoms 6 (V1) and 12 (V2 = second follow-up) weeks after COVID-19 infection, as assessed by systematic cardiopulmonary follow-up in an observational prospective multicentre trial.
This diagram was published by Thomas Sonnweber and Sabina Sahanic in the European Respiratory Journal, 2020.
Summary
The overlap between the medical specialisations in the department creates tremendous synergies. Our strong clinical and scientific background enables us to combine basic and clinical research and to gain new knowledge in both areas. Our aim is to personalise and optimise diagnostic tools, to refine the classification of diseases and the prognostic assessment of patients, and to improve treatment strategies in infectious diseases, clinical immunology, pneumology and rheumatology.
Selected Publications
Infectious Diseases
- Nairz M, Metzendorf C, Vujic-Spasic M, Mitterstiller AM, Schroll A, Haschka D, Hoffmann A, Von Raffay L, Sparla R, Huck CW, Talasz H, Moser PL, Muckenthaler MU, Weiss G.: Cell-specific expression of Hfe determines the outcome of Salmonella enterica serovar Typhimurium infection in mice. Haematologica. Oct 13; 2020 doi: 10.3324/haematol.2019.241745.
- Riedelberger M, Penninger P, Tscherner M, Seifert M, Jenull S, Brunnhofer C, Scheidl B, Tsymala I, Bourgeois C, Petryshyn A, Glaser W, Limbeck A, Strobl B, Weiss G, Kuchler K.: Type I Interferon Response Dysregulates Host Iron Homeostasis and Enhances Candida glabrata Infection. Cell Host Microbe. 27:454-466, 2020
- Pizzini A, Kurz K, Santifaller J, Tschurtschenthaler C, Theurl I, Fuchs D, Weiss G, Bellmann-Weiler R.: Assessment of neopterin and indoleamine 2,3-dioxygenase activity in patients with seasonal influenza: A pilot study. A Influenza Other Respir Viruses. 13:603-609, 2019
Iron Homeostasis
- Petzer V, Tymoszuk P, Asshoff M, Carvalho J, Papworth J, Deantonio C, Bayliss L, Wake MS, Seifert M, Brigo N, Valente de Souza L, Hilbe R, Grubwieser P, Demetz E, Dichtl S, Volani C, Berger S, Böhm F, Hoffmann A, Pfeifhofer-Obermair C, von Raffay L, Sopper S, Arndt S, Bosserhoff A, Kautz L, Perrier P, Nairz M, Wolf D, Weiss G, Germaschewski V, Theurl I.: A fully human anti-BMP6 antibody reduces the need for erythropoietin in rodent models of the anemia of chronic disease. Blood. 2020 Aug 27;136(9):1080-1090. doi: 10.1182/blood.2019004653.
- Demetz E, Tymoszuk P, Hilbe R, Volani C, Haschka D, Heim C, Auer K, Lener D, Zeiger LB, Pfeifhofer-Obermair C, Boehm A, Obermair GJ, Ablinger C, Coassin S, Lamina C, Kager J, Petzer V, Asshoff M, Schroll A, Nairz M, Dichtl S, Seifert M, von Raffay L, Fischer C, Barros-Pinkelnig M, Brigo N, Valente de Souza L, Sopper S, Hirsch J, Graber M, Gollmann-Tepeköylü C, Holfeld J, Halper J, Macheiner S, Gostner J, Vogel GF, Pechlaner R, Moser P, Imboden M, Marques-Vidal P, Probst-Hensch NM, Meiselbach H, Strauch K, Peters A, Paulweber B, Willeit J, Kiechl S, Kronenberg F, Theurl I, Tancevski I, Weiss G.: The haemochromatosis gene Hfe and Kupffer cells control LDL cholesterol homeostasis and impact on atherosclerosis development. Eur Heart J. 2020 Oct 21;41(40):3949-3959. doi: 10.1093/eurheartj/ehaa140.
- Haschka D, Volani C, Stefani A, Tymoszuk P, Mitterling T, Holzknecht E, Heidbreder A, Coassin S, Sumbalova Z, Seifert M, Dichtl S, Theurl I, Gnaiger E, Kronenberg F, Frauscher B, Högl B, Weiss G.: Association of mitochondrial iron deficiency and dysfunction with idiopathic restless legs syndrome. Mov Disord. 34:114-123, 2019.
Rheumatology
- Haschka D, Tymoszuk P, Bsteh G, Petzer V, Berek K, Theurl I, Berger T, Weiss G. Expansion of Neutrophils and Classical and Nonclassical Monocytes as a Hallmark in Relapsing-Remitting Multiple Sclerosis. Front Immunol. 2020 Apr 29;11:594. doi: 10.3389/fimmu.2020.00594
- Van Hoovels L, Broeders S, Chan EKL, Andrade L, de Melo Cruvinel W, Damoiseaux J, Viander M, Herold M, Coucke W, Heijnen I, Bogdanos D, Calvo-Alén J, Eriksson C, Kozmar A, Kuhi L, Bonroy C, Lauwerys B, Schouwers S, Lutteri L, Vercammen M, Mayer M, Patel D, Egner W, Puolakka K, Tesija-Kuna A, Shoenfeld Y, de Sousa MJR, Hoyos ML, Radice A, Bossuyt X.: Current laboratory and clinical practices in reporting and interpreting anti-nuclear antibody indirect immunofluorescence (ANA IIF) patterns: results of an international survey. Auto Immun Highlights. 2020 Nov 23;11(1):17. doi: 10.1186/s13317-020-00139-9.
- Camellino D, Duftner C, Dejaco C. New insights into the role of imaging in polymyalgia rheumatic. Rheumatology (Oxford). 2020 Nov 17:keaa646. doi: 10.1093/rheumatology/keaa646. PMID: 33200216 Oct 1;58(10):1802-1811.
Pneumology
- Thomas Sonnweber T, Sahanic S, Pizzini A, Widmann G, Luger A, Schwabl C, Kurz K, Koppelstätter S, Haschka D, Petzer V, Sonnweber B, Boehm A, Aichner M, Tymoszuk P, Lener D, Theurl M, Lorsbach-Köhler A, Tancevski A, Schapfl A, Schaber M, Hilbe R, Nairz M, Puchner B, Hüttenberger D, Tschurtschenthaler C, Aßhoff M, Peer A, Hartig F, Bellmann R, Joannidis M, Gollmann-Tepeköylü C, Holfeld J, Feuchtner G, Egger A, Hörmann G, Schroll A, Fritsche G, Wildner S, Bellmann-Weiler R, Kirchmair R, Helbok R, Prosch H, Rieder D, Trajanoski Z, Kronenberg F, Wöll E, Weiss G, Löffler-Ragg J, Ivan Tancevski I.: Cardio-pulmonary recovery after COVID-19 – an observational prospective multi-center trial. Eur Respir J. 2020 Sep
- Sahanic S, Boehm A, Pizzini A, Sonnweber T, Aichner M, Weiss G, Loeffler-Ragg J, Tancevski I.: Assessing self-medication for obstructive airway disease during COVID-19 using Google Trends. Eur Respir J. 2020 Nov 19;56(5):2002851. doi: 10.1183/13993003.02851-2020. Print 2020 Nov.
- Pizzini A, Aichner M, Sonnweber T, Tancevski I, Weiss G, Löffler-Ragg J.: The Significance of iron deficiency and anemia in a real-life COPD cohort. Int J Med Sci. 17:2232-2239, 2020
Selection of Funding
- Austrian National Bank Fund (Project 17271), Judith Löffler-Ragg
- Christian Doppler Society,-Christian Doppler Laboratory for Iron Metabolism and Anemia research, Günter Weiss
- Austrian Research Funds (FWF)-Doctoral Programm (HOROS—W-1253), Günter Weiss
- ERA-INFECT –FWF Joint Project (I-3321) , Günter Weiss
- ACOVACT (Austrian Ministry of Science), Günter Weiss
- Investigator-Initiated Study (IIS) grant by Boehringer Ingelheim (IIS 1199-0424), Ivan Tancevski
- Austrian Research Funds (FWF) Project P 28302, Igor Theurl
- Austrian Research Funds (FWF) Project P 33062, Manfred Nairz
- Austrian Society of Hematology and Medical Oncology (OeGHO), Verena Petzer
- Krebshilfe Tirol Project 15024, Piotr Tymoszuk
Collaborations
- Sarah H. Atkinson, Kenya Medical Research Institute (KEMRI) Centre for Geographic Medicine Coast, KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya
- Andreas Bergthaler, CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, Vienna, Austria
- Christian Bogdan, Institute of Clinical Microbiology, Immunology and Hygiene, Universitätsklinikum Erlangen and Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
- Christian Dejaco, Department of Rheumatology, Hospital of Brunico (SABES-ASDAA), Bruneck, Italy
- Ferric C. Fang, Departments of Laboratory Medicine and of Microbiology, University of Washington, Seattle, WA, USA
- Karl Kuchler, Max Perutz Laboratories, Center of Medical Biochemsitry, Medical University of Vienna, Austria
- Martina U. Muckenthaler, Department of Pediatric Hematology, Oncology and Immunology, University of Heidelberg, Heidelberg, Germany
- Josef M. Penninger, Department of Medical Genetics, Life Sciences Institute, University of British Columbia, Vancouver, BC, Canada
- Peter Pramstaller, Institute for Biomedicine, EURAC, Bozen, Italy
- Uwe Platzbecker, Department of Hematology, Cellular Therapy and Hemostaseology, Leipzig University Hospital, Leipzig, Germany
- Filip K. Swirski, Center for Systems Biology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
 Univ.-Prof. Dr.med.univ. Günter Weiss
Univ.-Prof. Dr.med.univ. Günter Weiss
Director
Contact:
Anichstraße 35
6020 Innsbruck
Austria
Email: guenter.weiss@i-med.ac.at
Phone: +43 512 504 23251
Fax: +43 512 504 23317
https://inneremed2.tirol-kliniken.at



