Biochemical Pharmacology
Research Focus
General Facts
Research
Selected Publications
Selected Funding, Collaboration
Keywords: Ion Channels, Molecular Biology, Biophysics, Physiology, Excitation-Contraction Coupling, Pharmacology, Zebrafish and mouse models, Inflammation, Toll-like receptors, Drug discovery
Research (ÖSTAT Classification) : 301206, 106023, 106006, 301213, 301902
Research Focus
Grabner-Dayal Laboratory
The Grabner-Dayal laboratory focuses on structural-functional studies into the excitation-contraction (EC) coupling machinery in the skeletal muscle, using the zebrafish and the mouse as model organisms. Another focus of the laboratory is on the role of the Ca2+-activated Cl- channel in skeletal muscle function.
Sandra Santos-Sierra Laboratory
We want to understand the molecular mechanisms underlying the innate immune response in the case of inflammatory diseases. Based on this knowledge, we intend to develop novel substances that can be applied specifically to modulate immune activity in pathologies with an inflammatory background (e.g. cancer, epilepsy).
General Facts
Grabner-Dayal Laboratory
The core mechanism of skeletal muscle contraction involves excitation of the sarcolemma, leading to release of Ca2+ from intracellular SR stores. This complex process, known as excitation-contraction (EC) coupling, involves the close interaction of two distinct Ca2+ channels: the voltage-gated L-type Ca2+ channel or dihydropyridine receptor (DHPR) present on the sarcolemma, and the intracellular Ca2+ release channel or ryanodine receptor (RyR1) in the SR. Our main research aim is to elucidate the structure-function relationship of this fascinating bidirectional Ca2+ channel crosstalk in skeletal muscle. We have mapped the functional domains in the DHPR α1S and β1a subunits that are crucial for this protein-protein signal transduction. Another interesting focus of our research is on understanding the duality of the DHPR as the voltage-sensor and Ca2+ channel, using phylogenetic approaches and with the involvement of a newly identified Ca2+-activated Cl- channel in this process.
Santos-Sierra Laboratory
The innate immune system plays a crucial role not only in fighting infections but also in numerous diseases and pathological conditions including cancer. Toll-like receptors (TLRs) are major components of this system. They recognise pathogens by means of ligation of pathogen-associated molecular patterns (PAMPS) and they recognise also host derived ligands resulting from tissue damage (damage-associated molecular patterns, DAMPS). The central role of TLRs in various inflammatory processes and in sepsis is well known. Thus, the discovery of substances with modulatory TLR signalling activity may have important implications in the treatment of a broad spectrum of pathologies linked to inflammation.
Research
Grabner-Dayal Laboratory
Recent major achievements: With our unique ki mouse model (DHPR(N617)), we have shown that the DHPR Ca2+ influx in the mammalian skeletal muscle – which was under investigation for more than half a century – is (very surprisingly) an evolutionary remnant (Dayal et al., Nat. Commun. 2017). In a follow-up study, we characterised the permeability and selectivity properties of the DHPR and hence the mechanism of Ca2+ non-conductance of the DHPR(N617) channel (Fig. 1).
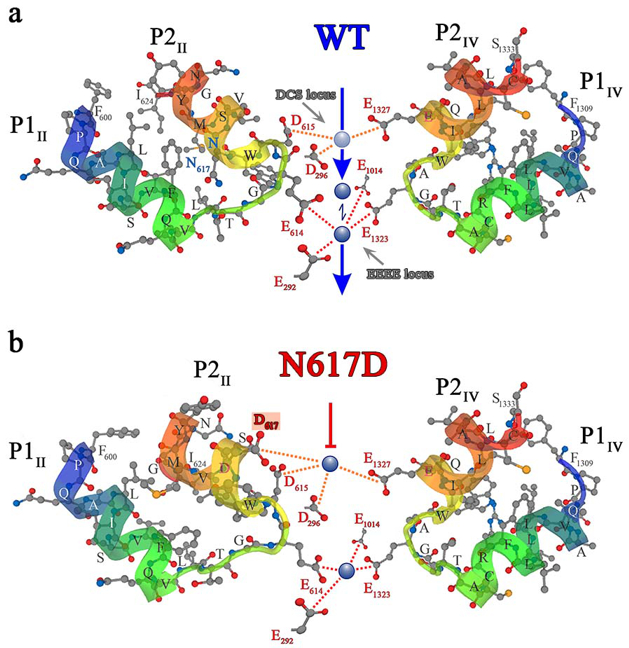
Fig. 1: Ca2+ selectivity and conductance mechanisms in the wt and mutant DHPR (N617D) channel pore. Conformational prediction models depicting the hypothetical mechanism of Ca2+ conductance through the wt DHPR (a) and the block of Ca2+ conductance due to atypical high Ca2+ binding affinity (as a result of introduction of the negative charge D617; boxed in red) in the DHPR(N617D) pore region. Dotted lines indicate binding interactions between Ca2+ ions (blue spheres) and carboxyl oxygens (red balls) of glutamates and aspartates. Low-affinity Ca2+ binding is indicated by light blue spheres and high affinity binding by dark blue spheres. The DCS locus is the divalent cation selectivity filter and the EEEE locus is the Ca2+ selectivity filter. Vertical blue arrows indicate an active Ca2+ conductance pathway in wt DHPR (a), and the red T-bar indicates a block of Ca2+ flux by aberrant high-affinity binding to the DCS locus in the mutant DHPR(N617D) channel pore (b).
In a recent study, we detected an intriguing physiological and biophysical difference in EC coupling between mammalian and highly evolved euteleost (bony fish) skeletal muscle: the unexpected participation of a Ca2+-activated Cl- channel (TMEM16A/ANO1). Using state-of-the-art approaches, we showed that ANO1 currents accelerate the repolarisation phase of skeletal muscle action potential (AP), resulting in slimmer APs (Fig. 2) and thus enabling the more closely controlled, faster and stronger muscle contractions that are crucial for high-speed swimming in the aquatic prey-predator environment. Phylogenetically, these novel findings provide an in-depth understanding of the evolution of skeletal muscle EC coupling.
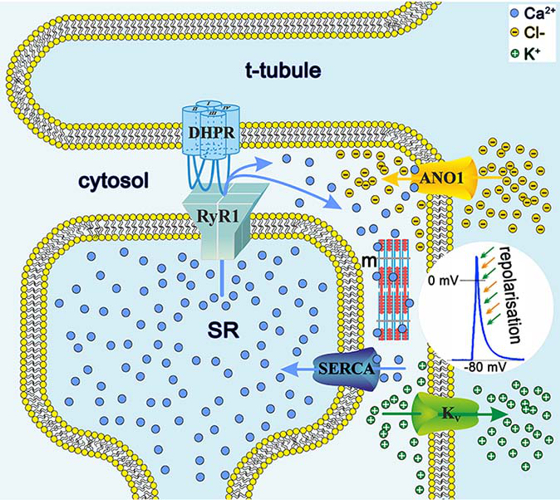
Fig. 2: Model of ANO1 activation by SR Ca2+ release as the basis for acceleration of AP repolarisation. Schematic representation of the skeletal muscle triad with sarcolemmal t-tubular invagination (t-tubule) adjacent to the sarcoplasmic reticulum Ca2+ store (SR), and membrane localisation of some selected channels and pumps involved in cytosolic (cytosol) Ca2+ handling. Depolarisation-induced conformational changes in the DHPR are initially transmitted to the RyR1, which leads to pore opening and to the release of Ca2+ ions (blue spheres) from the SR stores. Cytosolic Ca2+ activates contraction of the muscle fibres (m) and binds to intracellular ANO1 Ca2+ binding sites, causing massive Cl− influx (yellow spheres) into the cytosol. Together with simultaneous K+ efflux (green spheres) via the voltage-gated K+ channels (KV), Cl− influx synergistically and rapidly reduces the membrane potential to accelerate AP repolarisation (inset green and yellow arrows). Finally, the SERCA pumps Ca2+ back into the SR and thus resets the system.
Another focus of our research is on the identification of molecular regions of the β1a subunit of the DHPR complex, which is essential for ultrastructural arrangement of DHPR in the sarcolemma (tetrads).
Santos-Sierra Laboratory
The therapeutic potential of TLRs as targets in a variety of inflammatory diseases is being steadily explored. In particular, Toll-like receptor 2 (TLR2) recognises bacterially derived di- and tri-acylated lipopeptides and additionally initiates an inflammatory response following recognition of endogenous host ligands produced during cellular stress and tissue damage (e.g. HMGB1, hyaluronan). Our group has discovered various compounds, synthetic small-molecules (Murgueitio, MS. et al. 2014 and 2017) and peptides (Ebner, S. et al. 2018) that modulate TLR2 receptor activity and other inflammatory pathways, such as IL-1R and TNF-R pathways (Fig. 3; Drexel, M. et al. 2019, and manuscript in preparation). At present, we are testing the efficacy of the inhibitory compounds in several relevant disease models (Wietzorrek, G. et al. 2019, and manuscript in preparation).
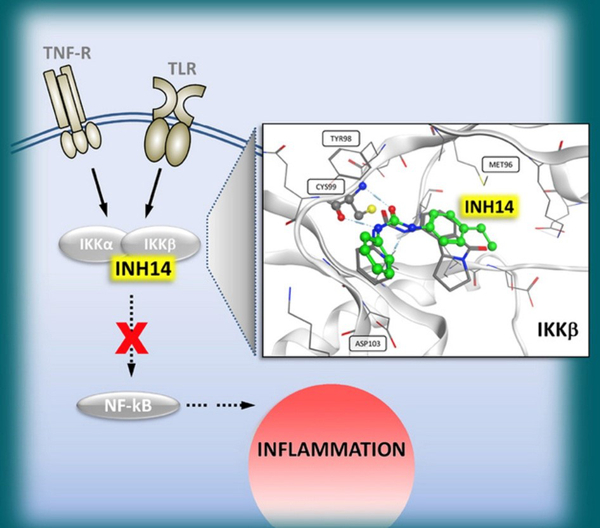
Fig. 3: INH14 is a cell‐permeable urea derivative, N‐(4‐ethylphenyl)‐N′‐phenylurea, which is able to reduce the proinflammatory activity induced by IKK‐dependent Toll‐like receptors, IL‐1R and TNF‐R by targeting IKKα/β. Optimisation of INH14 could produce potent inhibitors of IKKs that might be used as anti‐inflammatory drugs (Cover feature, ChemBioChem, May 2019).
Selected Publications
- Dayal, Anamika; Ng, Shu Fun J.; Grabner, Manfred: Ca2+-activated Cl- channel TMEM16A/ANO1 identified in zebrafish skeletal muscle is crucial for action potential acceleration. NATURE COMMUNICATIONS. 2019; 10(1); 115. doi: 10.1038/s41467-018-07918-z.
- Idoux, Romane; Fuster, Clarisse; Jacquemond, Vincent; Dayal, Anamika; Grabner,Manfred; Charnet, Pierre; Allard, Bruno: Divalent cations permeation in a Ca2+ non-conducting skeletal muscle dihydropyridine receptor mouse model. CELL CALCIUM. 2020; 91:102256. doi: 10.1016/j.ceca.2020.102256.
- Dayal, Anamika and Grabner, Manfred: Pore mutation N617D in the skeletal muscle DHPR blocks Ca2+ influx due to atypical high affinity Ca2+ (in revision)
- Developments in anticancer vaccination: budding new adjuvants. Santos-Sierra S. Biol Chem. 40:435-446 (2020).
- Anti-inflammatory activity of small-molecule antagonists of Toll-like receptor 2 (TLR2) in mice. Wietzorrek G, Drexel M, Trieb M, Santos-Sierra S. 224(1):1-9 (2019).
- Cover Feature: INH14, a Small‐Molecule Urea Derivative, Inhibits the IKKα/β‐Dependent TLR Inflammatory Response. Drexel M, Kirchmair J, Santos‐Sierra S. ChemBioChem (2019)
- INH14, a Small-Molecule Urea Derivative, Inhibits the IKKα/β-Dependent TLR Inflammatory Response. Drexel M, Kirchmair J, Santos-Sierra S. 20:710-717 (2019).
Selection of Funding
- 2014-2019, Austrian Science Fund (FWF) P27392-B21; Structure – Functional Link in the DHPR ß1a Subunit for Tetrad Formation and Skeletal Muscle Motility.
- P30779-FWF: Modulation of inflammation in epilepsy. 2018-2022. Principal investigator Priv. Doz. Dr. Meinrad Drexel (Inst. Mol. and Cellular Pharmacology), collaborator: Priv. Doz. Dr. Sandra Santos Sierra.
Collaborations
- Prof. Clara Franzini-Armstrong: Department of Cell and Developmental Biology, Univ. of Pennsylvania, Philadelphia, U.S.A.
- Prof. Kurt Beam, Department of Physiology and Biophysics, University of Colorado Anschutz Medical Campus, U.S.A.
- Prof. Bruno Allard, Institut NeuroMyoGène, University of Lyon, France.
- Prof. Mark Rich, Department of Neuroscience, Wright State University, Dayton, U.S.A.
- Dr. Werner Melzer: Department of Molecular Physiology and Biophysics, Universität ULM, Deutschland.
- Dr. Xaver König, Center for Physiology and Pharmacology, Medical University of Vienna, Austria.
- Prof. Johannes Kirchmair: Computational Drug Discovery and Design Group. University of Vienna. Vienna. Austria.
- Prof. Philipp Henneke: Center for Chronic Immunodeficiency. Faculty of Medicine Albert-Ludwigs-universität Freiburg. Freiburg. Germany.
- Priv. Doz. Florian Lagler: Research Institute of inborn errors of metabolism. Paracelsus Medical University Salzburg. Salzburg. Austria.
- Prof. Darek Gorecki: Institute of Biological and Biomedical Sciences. Faculty of Science and Health. University of Portsmouth. Portsmouth. UK.
 Univ.-Prof. Dr.med.univ. Hans-Günther Knaus
Univ.-Prof. Dr.med.univ. Hans-Günther Knaus
Director
Contact:
Peter Mayer Straße 1
6020 Innsbruck
Austria
Email: Hans.G.Knaus@i-med.ac.at
Phone: +43 512 9003 70440
Fax: +43 512 9003 73440
https://www.i-med.ac.at/ibp/



