Internal Medicine I
Research Focus
General Facts
Research
Selected Publications
Selected Funding, Collaboration
Keywords: Gastroenterology, endocrinology, metabolism, inflammation, hepatology, nutrition, non-alcoholic fatty liver disease, inflammatory bowel disease, microbiota
Research (ÖSTAT Classification) : 3518, 3510, 3516, 3536, 3509
Research Focus
The department focuses on translational research in the fields of gastroenterology, hepatology, endocrinology and metabolism, and it is represented by several research groups. The overall objective of our scientific activities is to gain improved insights into the pathophysiology of prevalent diseases, such as inflammatory bowel disease, metabolic and genetic liver diseases, obesity, type 2 diabetes and atherosclerosis. Better knowledge will help to improve clinical management of these patients in the future.
The major research topics are:
- The causes and effects of intestinal inflammation, particularly in connection with diseases such as Crohn’s disease and ulcerative colitis
- The role of inflammation, microbiome and metabolism in type 2 diabetes and non-alcoholic fatty liver disease
- Genetic liver disease
- Molecular effects of environmental factors in obesity and insulin resistance, and the interaction of multiple organs or tissues in metabolic disease
- Mechanisms in atherosclerosis
General Facts
The Department of Internal Medicine I focuses on the specific medical areas of gastroenterology, hepatology, endocrinology and metabolism. Our division has around 45 employees, and many members including students are involved both in clinical work and in research. Our laboratories are excellently equipped and our researchers are able to conduct state-of-the-art research in the field of cellular and molecular work. The common aims of our research activities are to improve knowledge in the respective disease areas and to improve patient care.
In our department we are host to three Christian Doppler research laboratories:
- Christian Doppler research laboratory on mucosal immunology (Univ.-Prof.Dr. Moschen )
- Christian Doppler research laboratory on insulin resistance (Univ.-Prof.in Dr.in Kaser)
- Christian Doppler research laboratory on iron and phosphate biology (Univ.-Prof.Dr. Zoller)
For a number of years, our research has been funded by the Austrian Research Promotion Agency (FFG), Austrian Science Fund (FWF), Christian Doppler Research Association (CDG) and European Union (FP7). We have published our research in highly respected international journals, such as Nature Reviews Immunology, Nature Communications, New England Journal of Medicine, Cell Host Microbe, Gastroenterology, Gut, JAMA and many others.
Research
Microbiota and Gastrointestinal Disorders
Herbert Tilg
This research group has been involved in microbiome research for over a decade. Several exciting projects have been completed, for example demonstrating a key role of Akkermansia muciniphila in alcoholic liver disease (Grander C. Gut 2018; 67: 891). The following microbiome projects are currently in progress:
- Inflammatory bowel diseases: the role of gut microbiome in treatment responses (Effenberger M et al. J Crohns Colitis 2021)
- The role of gut and liver microbiome in hepatocellular carcinoma (in collaboration with K. Aden, Kiel, Germany)
- Gut microbiome in the Bruneck cohort (in collaboration with J. Raes, Leuven, Belgium)
- CHRIS study: Type 2 diabetes and NAFLD: pathophysiological relevance of gut microbiome and metabolites (study conducted in collaboration with EURAC and V. Temaroli, Gothenburg, Sweden)
Intestinal Inflammation
Timon Adolph
In the Department of Internal Medicine I, we are interested in inflammatory diseases of the gastrointestinal tract and particularly in understanding the pathophysiology of inflammatory bowel disease. In a stand-alone project funded by the FWF, we identified the role of intestinal epithelial glutathione peroxidase 4 (GPX4) in intestinal homeostasis. We discovered that a Western diet over a period of 3 months induces a phenotype similar to Crohn’s disease in GPX4-deficient mice. In our work, published in Nature Communications (Mayr L. et al., Nature Communications 2020, 11, 1775), we report that GPX4 restricts a cytokine response –specifically interleukin 6 (IL-6) and C-X-C Motif Chemokine Ligand 1 (CXCL1) expression – from intestinal epithelial cells (IECs), which is elicited by dietary polyunsaturated fatty acids (PUFAs). In line with this observation, we noted that mice with a lack of one Gpx4 allele specifically in IECs developed small intestinal inflammation that closely resembled Crohn’s disease when exposed to a PUFA-enriched Western diet. As such, we have identified that dietary PUFAs (contained in meat, eggs and oils) can trigger a phenotype similar to Crohn’s disease in mice with reduced epithelial GPX4 activity. Our study may also contribute to the dietary advice provided to IBD patients, which needs to be established in future clinical trials.
This project ran from 1st August 2016 to the 30th September 2020 and employed 2 PhD students, who shared their first author contribution in the published work. The project also funded the work of 3 MD students, who undertook their diploma theses in our laboratory and who contributed to the key publication (Mayr L. et al., Nature Communications 2020, 11, 1775). With the support of the MUI, all the national and international researchers involved in this project gained new scientific insights and developed their academic careers, as demonstrated by our key open-access publication, which was also promoted in a press release and on the MUI homepage. Following this, German ( “Der Spiegel”, “Radio Berlin Brandenburg” and the TV service “Bayrischer Rundfunk”) and Austrian (“ORF”, “Tiroler Tageszeitung” and “Scilog”) news outlets reported on our study in their scientific sections.
Based on this success, we were able to secure further funding. The laboratory has now received support from the Austrian Society for Gastroenterology and Hepatology and from the FWF, for mechanistic deciphering of the phenotype similar to Crohn’s disease in mice. In future studies, we will use this model to explore the role of the intestinal microbiota and epithelial signalling hubs previously implicated in IBD.
Hepatology
Heinz Zoller
Specific research projects focus on rare haemochromatosis variants, congenital iron metabolism defects that result in liver disease. One of these rare haemochromatosis variants is ferroportin disease, which is caused by autosomal dominant mutations in SLC40A1. It was previously unknown why humans who have inactivating ferroportin mutations should present with iron overload. A recent molecular and translational research study conducted in our laboratory then showed that specific disease-related ferroportin variants not only cause reduced iron export activity in steady state but are also resistant to inactivation by the physiological inhibitor of ferroportin, hepcidin, which is a peptide hormone (Viveiros et al., Liver Int 2020; 40: 1941 – 1951). These findings provide an explanation of the disease mechanism in which, in states of high circulating hepcidin concentration, iron transport by means of ferroportin is increased relatively as a result of its resistance to the peptide hormone. In conclusion, our research shows that the relationship between classical HFE-related haemochromatosis and ferroportin disease is comparable with type 1 and type 2 diabetes, where deficiency in the former as well as resistance to the respective peptide hormones – insulin in the case of diabetes and hepcidin in the case of haemochromatosis – cause the disease.
One emerging research focus of the hepatology group was prompted by the clinical observation that patients with inflammatory bowel disease, who were treated repeatedly with the specific intravenous iron formulation ferric carboxymaltose, developed symptomatic hypophosphataemia. Our research into iron and phosphate biology is now funded by Pharmacosmos and the Christian Doppler society and conducted in the recently established Christian Doppler Laboratory on Iron and Phosphate Biology. To examine whether the risk of hypophosphataemia was lower in patients treated with a more advanced intravenous iron formulation, a prospective randomised controlled trial was conducted, which compared ferric carboxymaltose with the newer formulation, iron isomaltoside. This study showed that, in patients with iron deficiency, only 8% of patients treated with iron isomaltoside developed mild to moderate hypophosphataemia – all of whom recovered after 7 weeks. By contrast, over 74% of patients treated with ferric carboxymaltose developed hypophosphataemia, in 11% of which patients this was severe and in 45% of which patients the phosphate persisted beyond the 7-week study duration (Wolf et al., JAMA 2020; 323: 432 – 443).
To assess why this side effect can cause long-term and sometimes irreversible complications, a comprehensive range of mineral and bone metabolism parameters has been included. In line with the clinical observations, hypophosphataemia in patients treated with ferric carboxymaltose was caused by high concentrations of the peptide hormone FGF23, which triggered a decrease in active vitamin D, hypocalcaemia and secondary hyperparathyroidism. To summarise the complex changes elicited by ferric carboxymaltose in patients with iron deficiency, we recently coined the term ‘6H syndrome’(Schaefer et al., Mol Aspects Med 2020; 75: 100862).
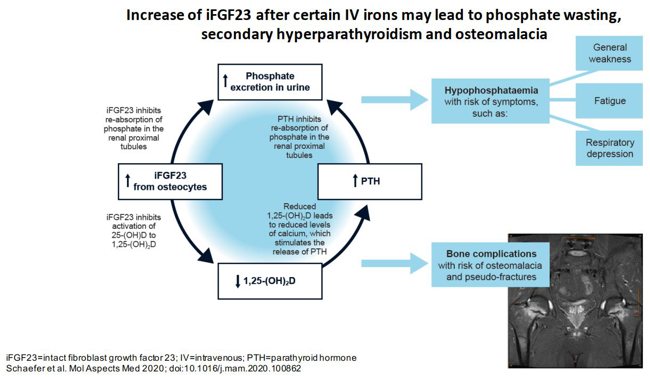
Fig. 1: Certain intravenous iron formulations cause an increase in concentrations of the peptide hormone FGF23 in blood. This triggers a cascade of biochemical and endocrine changes, with the clinical result of increased bone fragility, hypophosphataemic osteomalacia. The description ‘6H syndrome’ has been coined to summarise these changes.
Endocrinology and Metabolism
Pathophysiology of Insulin Resistance and Type 2 Diabetes
Diabetes mellitus is one of the top ten causes of death worldwide and it affects more than 800,000 people in Austria, most of whom suffer from type 2 diabetes. Another 300,000 people in Austria are thought to suffer from prediabetes and are therefore at very high risk of being diagnosed with diabetes within the next few years. As well as a strong genetic predisposition, overnutrition, a sedentary lifestyle and smoking are well-known risk factors for type 2 diabetes.
One major focus of our research was on defining which diets are especially harmful from a metabolic perspective and what the underlying molecular mechanisms are. In short, we found that frequently consumed Western diets –defined as high-fat, high-sucrose and cholesterol-enriched diets – have the most detrimental effects, especially on adipose tissue but also on the liver. The consumption of fructose-enriched diets is primarily associated with liver damage and inflammation in adipose tissue, although the harmful effects on adipocyte morphology and insulin sensitivity were less pronounced when compared with high-fat diets. In a follow-up project, we investigated switching the diet from a harmful, Western diet to a more beneficial, standard diet, without restricting calorie intake. In this work (paper submitted), we showed that changing the diet without pronounced weight reduction partly restores thermogenic capacity or reverses inflammation and alterations in adipocyte morphology.
Pathophysiology and Treatment of Diabetes-Related Organ Damage
Diabetes mellitus is associated with several long-term complications, such as coronary heart disease, peripheral artery disease, neuropathy, retinopathy and fatty liver disease as well as chronic kidney disease and heart failure. Only recently, a novel class of antidiabetic drugs (SGLT2 inhibitors) was found to exert glucose-independent protective effects on hospitalisation for heart failure and on the development or progression of chronic kidney disease. The underlying protective mechanisms are not yet fully understood. We showed that even short-term SGLT-2 inhibitor therapy improves the overactivation of cardiac insulin signalling, which is thought to be a major driver of heart failure in diabetics. Additionally, we found that short-term SGLT-2 inhibitor therapy improves mitochondrial mass and structure independently of its effects on body weight. These studies provide novel cardioprotective mechanisms of SGLT-2 inhibitors and reveal novel treatment targets in diabetic cardiomyopathy. In an ongoing study, we are investigating whether long-term empagliflozin supplementation prevents metabolic diseases, such as fatty liver disease, insulin resistance, obesity and related end-organ damage.
Infection Risk in Diabetes
The relationship between BMI and infection risk is sometimes controversial and it is U-shaped for certain infections. While it is well known that diabetes is associated with an increased risk of specific infections (especially respiratory, skin and urogenital infections), the underlying mechanisms and their contributions are not fully defined; hyperglycaemia, end-organ damage and obesity are discussed as major drivers of increased infection risk. During the Covid-19 pandemic, it emerged that diabetics with SARS-CoV2 infections have an increased risk of ICU admission and that mortality increased significantly in diabetic patients. However, it was unclear which specific patients were affected by very high risk. Data from a national registry kept by the Austrian Diabetes Association suggested that patients with diabetes in old age, chronic kidney disease, peripheral artery disease and elevated CRP and AST levels are at very high risk of death as a result of SARS-CoV2 infection. Such very high risk was not restricted to patients with overt diabetes but rather also seen in patients with prediabetes.
Atherosclerosis
Andreas Ritsch
We are interested in the role of reverse cholesterol transport in atherosclerosis. Recently, we investigated structure-function-disease relationships of HDL in healthy subjects and in patients with diabetes (T2DM) or coronary heart disease (CHD). We showed that different cellular functions of HDL have a weak correlation with one another and are determined by different structural components (Cardner M, et al., JCI Insight. 2020 Jan 16; 5(1): e131491). In pharmacological studies of ApoE knockout rabbits, an animal model for atherosclerosis, we showed that Matcha green tea enhances atherosclerosis in these animals, due to impaired reverse cholesterol transport (Monika Hunjadi, et al., manuscript submitted). In a large clinical study (Young Finns study), we showed an inverse correlation between HDL cholesterol efflux capacity and subclinical cardiovascular risk markers in young adults (Hunjadi M, et al., Scientific Reports. 2020 Nov 5; 10(1): 19223.). Investigations in 2468 participants of the LURIC study showed that cholesterol efflux is associated with HDL composition as well as inflammatory burden in patients who have been referred for coronary angiography and that this is an inverse predictor of cardiovascular mortality, independently of HDL cholesterol (Ritsch A, et al., Biomedicines. 2020 Nov 21; 8(11): 524.).
Selected Publications
- Mayr, L.; Grabherr, F.; Schwarzler, J.; Reitmeier, I.; Sommer, F.; Gehmacher, T.; Niederreiter, L.; He, G.W.; Ruder, B.; Kunz, K.T.R., et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn's disease. Nature communications 2020, 11, 1775, doi:10.1038/s41467-020-15646-6.
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E.; The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol 2020, 20:40
- Grander, C.; Schaefer, B.; Schwarzler, J.; Grabherr, F.; dee Graaf, D.M.; Enrich, B.; Oberhuber, G.; Mayr, L.; Sangineto, M.; Jaschke, N.; Adolph, T.E.; Moschen, A.R.; Effenberger, M; Dinarello, C.A.; Zoller, H.; Tilg. H; Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018; 67:881-901.
- Sourij H et al & Kaser S. COVID-19 fatality prediction in people with diabetes and prediabetes using a simple score upon hospital admission. Diabetes Obes Metab 2020
- Radlinger B et al & Kaser S. Cardioprotective effects of short-term empagliflozin treatment in db/db mice. Sci Rep 2020
- Ress C et al & Kaser S. Apolipoprotein A5 controls fructose-induced metabolic dysregulation in mice. Nutr Metab Cardiovasc Dis, online
- Viveiros A, Panzer M, Baumgartner N, et al. Reduced iron export associated with hepcidin resistance can explain the iron overload spectrum in ferroportin disease. Liver Int 2020;40:1941-1951.
- Wolf M, Rubin J, Achebe M, et al. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials. JAMA 2020;323:432-443.
- Schaefer B, Meindl E, Wagner S, et al. Intravenous iron supplementation therapy. Mol Aspects Med 2020;75:100862.
- Cardner M, Yalcinkaya M, Goetze S, Luca E, Balaz M, Hunjadi M, Hartung J, Shemet A, Kränkel N, Radosavljevic S, Keel M, Othman A, Karsai G, Hornemann T, Claassen M, Liebisch G, Carreira E, Ritsch A, Landmesser U, Krützfeldt J, Wolfrum C, Wollscheid B, Beerenwinkel N, Rohrer L, von Eckardstein A. Structure-function relationships of HDL in diabetes and coronary heart disease. JCI Insight. 2020 Jan 16;5(1):e131491.
- Ritsch A, Duerr A, Kahler P, Hunjadi M, Stojakovic T, Silbernagel G, Scharnagl H, Kleber ME, März W. Cholesterol Efflux Capacity and Cardiovascular Disease: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Biomedicines. 2020 Nov 21;8(11):524.
- Hunjadi M, Lamina C, Kahler P, Bernscherer T, Viikari J, Lehtimäki T, Kähönen M, Hurme M, Juonala M, Taittonen L, Laitinen T, Jokinen E, Tossavainen P, Hutri-Kähönen N, Raitakari O, Ritsch A. HDL cholesterol efflux capacity is inversely associated with subclinical cardiovascular risk markers in young adults: The cardiovascular risk in Young Finns study. Sci Rep. 2020 Nov 5;10(1):19223. doi: 10.1038/s41598-020-76146-7.
Selection of Funding
- FWF No.: FT33070 (Timon Adolph)
- Christian-Doppler-Research Laboratory for mucosal immunology (Univ.-Prof.Dr. Moschen )
- Christian-Doppler-Research Laboratory for insulin resistance (Univ.-Prof.in Dr.in Kaser)
- Christian-Doppler-Research Laboratory for Iron and Phosphate Biology (Univ.-Prof.Dr. Zoller)
- Funding is also supported by the excellence initiative VASCage (Centre for Promoting Vascular Health in the Ageing Community), an R&D K-Centre (COMET program - Competence Centers for Excellent Technologies) funded by the Austrian Ministry for Transport, Innovation and Technology, the Austrian Ministry for Digital and Economic Affairs and the federal states Tyrol, Salzburg and Vienna. (Herbert Tilg)
Collaborations
- Prof. Arthur Kaser, Cambridge
- PD Dr. Konrad Aden, Kiel
- Prof. Richard Steven Blumberg, Boston Harvard Medical School, Gastroenterology, Hepatology & Endoscopy
- Prof. Michael Roden, Düsseldorf, Endocrinology
- Erwin Wagner, PhD, Medical University Vienna
- Prof. Michael Trauner, Medical University Vienna
- Prof. Percy A. Knolle, TUM Technical University Munich
 Univ.-Prof. Dr. med. univ. Herbert Tilg
Univ.-Prof. Dr. med. univ. Herbert Tilg
Director
Contact:
Anichstraße 35
6020 Innsbruck
Austria
Email: herbert.tilg@i-med.ac.at
Phone: +43 512 504 23539
Fax: +43 512 504 23538
Universitätsklinik für Innere Medizin I Innsbruck



