Internal Medicine III
Research Focus
General Facts
Research
Selected Publications
Selected Funding, Collaboration
Keywords: Cardiology, angiology, biosignals, COVID-19, myocardial infarction, magnetic resonance imaging, atherosclerosis, biomarker, cardiovascular repair, interventional cardiology
Research (ÖSTAT Classification) : 301109, 302006, 302030, 302032, 302071
Research Focus
The clinical research of our department mainly focuses on myocardial infarction and heart failure, advanced cardiac imaging technologies as well as biosignal analysis, digital medicine and electrophysiology. In all these areas, the department conducts or participates in large multicentre studies. The basic research of our department focuses on cardiovascular repair.
General Facts
Univ.-Prof. Dr. Axel Bauer has been head of the Department of Internal Medicine III, Cardiology & Angiology at the Medical University of Innsbruck since July 2019. He is an interventional cardiologist and clinical researcher, specialising in biosignal analysis and digital medicine.
In the department, research is structured in several groups with distinct research focuses, ranging from translational science to clinical research. The working group of Prof. A. Bauer mainly concentrates on characterisation of the different facets of the cardiac autonomic nervous system, by means of novel non-invasively derived biomarkers assessed from ECG signals, blood pressure recordings and respiratory signals. The aim is to improve the prognosis of patients through individualised management on the basis of this approach. Another principal research group is headed by Univ.-Prof. Dr. B. Metzler and focuses mainly on advanced imaging modalities in patients with acute myocardial infarction and valvular heart disease. Furthermore, the research group performs randomised control studies and is involved in large multicentre trials. Translational research with a primary focus on cardiovascular repair is conducted mainly by Univ.-Prof. Dr. M. Zaruba.
Research
Biosignal Analysis
Leader: Univ.-Prof. Dr. Axel Bauer
Members: Dr.in Theresa Dolejsi; Dr. Michael Schreinlechner; Dr. Lukas von Stülpnagel; Dr. Michael Toifl; Dr. Nikolay Vdovin
The working group on biosignal analysis investigates the cardiac autonomic nervous system (CAS), which provides important information on numerous cardiovascular diseases. To provide an insight into the CAS, biological signals influenced by the CAS are analysed; signals such as ECG, blood pressure, respiratory activity or oxygen saturation are obtained non-invasively from the body surface, using conventional or mobile devices. The complex nature of the signals requires the development of mathematical algorithms to reveal relevant information.
In recent years, the working group has developed major digital biomarkers, including deceleration capacity (DC) or periodic repolarisation dynamics (PRD), which have subsequently been validated in large international studies. Recently, in the EU-CERT-ICD study, a large European project involving 15 countries (NCT02064192), the working group demonstrated for the first time that a digital biomarker (PRD) was able to predict not only clinical outcomes but also the therapeutic effect of an implanted device with respect to mortality reduction. In the ongoing SMART-MI study, a prospective randomised control study performed in 31 centres across Germany and Austria (NCT02594488), the working group is testing whether implantable cardiac monitors are useful in selected post-infarction patients with CAS dysfunction, in order to detect serious arrhythmic events. Results are expected in mid-2021.
In current projects, the research group is working on the implementation and clinical application of digital biomarkers integrated into monitors in intensive care units. Another focus is on the integration of biomarkers into mobile devices, including smartphones and smartwatches. The group is also working on the production of a telemonitoring system for COVID-19 patients. In addition, the group is conducting a multicentre randomised control trial to investigate the effects of RAS inhibition on COVID-19; this is being undertaken at more than 30 centres in Austria and Germany.
Cardiac Magnetic Resonance Tomography and Myocardial Infarction
Leader: Univ.-Prof. Dr. Bernhard Metzler
Members: Priv.-Doz. Dr. Sebastian Reinstadler, PhD; Assoz.-Prof. Dr. Gert Klug; Assoz.-Prof.in Dr.in Agnes Mayr, Dr. Martin Reindl, PhD; Dr.in Christina Tiller; Dr.in Magdalena Holzknecht; Dr. Ivan Lechner, PhD Student; Dr. Felix Troger, PhD Student
The working group on “Cardiac Magnetic Resonance Tomography (CMR)”, led by Univ.-Prof. Dr. B. Metzler, MSc was founded in 2002 and has published over 133 original research papers in peer-reviewed journals since that time. One of its main focuses is on CMR imaging in patients with ST segment elevation myocardial infarction (STEMI), to obtain deeper insights into the pathophysiology of myocardial tissue injury, infarct healing and myocardial remodelling. The assessment of degenerative aortic valve stenosis and CMR-guided transcatheter aortic valve implantation (TAVI) planning is another important focus of the research group. The working group is also involved in randomised, multicentre, multinational clinical trials. The study group is based on close cooperation between the University Clinic for Internal Medicine III, Cardiology and Angiology (Head: Univ.-Prof. Dr. A. Bauer) and the Department of Radiology (Head: Univ.-Prof.in Dr.in E. Gizewski) at the Medical University of Innsbruck. Univ.-Prof. Metzler’s team consists of two associate professors, one senior physician, three fellows and two PhD students as well as a current total of 7 diploma students. Between 2018 and 2020, the working group published 24 peer-reviewed original research articles and obtained nine research grants, including the renowned “Hans-Blömer-Young Investigator Award”, the “Paracelsus prize” and the “Werner-Klein Research Prize”. International cooperation was initiated with the CMR working groups of Prof. Dr. H. Thiele at the University of Leipzig in Germany and Prof. Dr. I. Eitel at the University of Lübeck in Germany, with another seven publications. Future aims include the establishment of extended cooperation with centres in Austria, in order to perform randomised multicentre CMR studies.
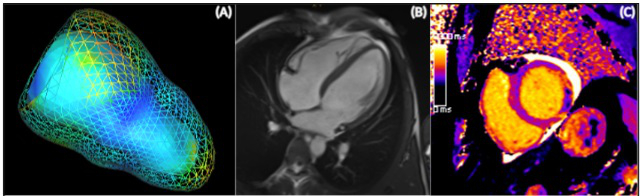
Fig. 1: Cardiac magnetic resonance approaches to estimate left ventricular strain (A), function (B) and inflammation (C).
Cardiovascular Repair and Aging
Leaders: Assoz. Prof. Priv. Doz. Dr. Marc-Michael Zaruba; Univ.-Prof. Dr. Gerhard Pölzl
Members: Moritz Messner, PhD
Our major research goal is the development of novel therapies based on inflammatory, chemokine-driven and aging-related mechanisms to treat ischaemic and non-ischaemic cardiomyopathies. Using genetic mouse models, we specifically investigate the impact of smooth muscle-specific knockout of the chemokine SDF-1 on cardiovascular development and regeneration. Moreover, we are interested in stimulating cardiac repair mechanisms based on the activation of HIF-1 target genes by prolyl hydroxylase inhibitors and in analysing aging-related splicing variants in patients with cardiomyopathy.
Research Projects:
- Cell-specific role of SDF-1 in the recruitment of progenitor cells after myocardial ischaemia
Ongoing research suggests a fundamental role of the SDF-1/CXCR4 axis in cardiac repair and tissue homeostasis after ischaemia. Nevertheless, there is still little understanding of the precise cellular mechanisms by which SDF-1-dependent cell migration is orchestrated. In our current FWF-funded project, we aim to identify important cellular sources of SDF-1-dependent cell recruitment and cardiac repair. Based on previous work and preliminary data, we aim to investigate the cell-specific effects of SDF-1 ablation in smooth muscle cells within the cardiovascular system, using conditional, SDF-1-specific knockout mouse models. Moreover, we elucidate the therapeutic potential of HIF prolyl hydroxylase inhibitors (PHI) to induce SDF-1 gene expression and stimulate cardiac repair in the ischaemic heart. Our data suggest that the inhibition of prolyl hydroxylase may be a promising target for HIF-1a-mediated SDF-1 activation, to increase stem cell homing and myocardial repair (see Fig. 2).
- Aging-related splicing variants in patients with cardiomyopathy
Defined mutations in the human lamin A gene or in enzymes that process the important nuclear membrane protein LMNA (e.g. Zmpste24) are causally involved in premature aging syndromes, such as progeria. Patients who suffer from progeria develop severe cardiovascular morbidities, such as stroke, myocardial infarction and severe atherosclerosis leading to early death.
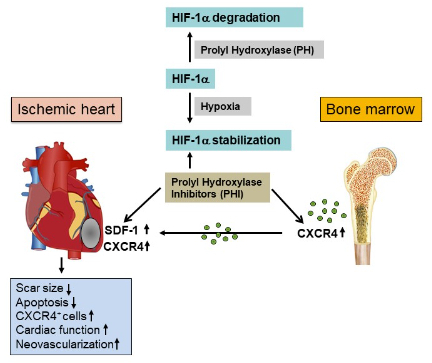
Fig. 2: Therapeutic concept of prolyl hydroxylase inhibition to enhance SDF-1 and CXCR4 expression for myocardial repair
Under normoxic conditions, HIF-1a protein is inactivated by prolyl hydroxylases, leading to HIF-1a degradation. Hypoxia or PHI leads to reduced prolyl hydroxylase activity, thus stabilising HIF-1a, which translocates to the nucleus and upregulates downstream target genes.
Clinical Trials in Interventional Cardiology
Leader: Univ.-Doz. Dr. Christoph Brenner
Interventional cardiology today represents one of most important subdisciplines in internal medicine. In particular, the minimally invasive treatment of acute myocardial infarction within the heart catheter laboratory is of the highest prognostic relevance. As a further optimisation of our diagnostic and therapeutic approaches, we have established a structured patient database, in order to conduct various forms of clinical study.
In recent randomised clinical trials, we have been able to introduce and investigate new and innovative therapeutic approaches, such as the administration of a DPP4 inhibitor in combination with G-CSF in the setting of acute ST elevation myocardial infarction for improved myocardial regeneration, a concept that is currently under further investigation in ongoing multicentre clinical trials. Other studies conducted in our clinical trials centre are investigating the latest coronary stent platforms for the treatment of coronary artery disease or optimised concomitant pharmacological treatment regimens. Another focus of our work is on improving the safety and efficacy of cardiac catheterisation.
Future projects of our group will help to optimise treatment pathways for acutely diseased cardiac patients in Austria and Europe.
Endothelial Cell Biology
Leader: Prof. Dr. R. Kirchmair
Members: Dr. Markus Theurl, PhD; Daniela Lener, B.Sc.
Our research group focuses on vascular cell biology. In previous studies, we have shown that administration of the neuropeptide secretoneurin via a gene therapeutic vector results in neovascularisation in various cardiovascular mouse models. This positive effect has now also been demonstrated in a diabetes mellitus mouse model, which may have therapeutic implications, as diabetics in particular often qualify neither for interventional nor for surgical revascularisation.
A second focus of our research group is on the influence of tyrosine kinase inhibitors (for the treatment of chronic myeloid leukaemia) on endothelial cell function. This is due to clinical observations that patients treated with nilotinib and ponatinib showed an accumulation of cardiovascular events. In a translational study, in cooperation with colleagues from Vienna General Hospital, our group was able to show that nilotinib interferes with lipid metabolism, leads to an increase in LDL and has a pro-atherogenic effect.
In a clinical study on patients without manifest cardiovascular disease, we were able to show that elevated levels of the acute phase protein lipocalin-2 are detectable even with a low plaque burden in the carotid and femoral artery in serum. Future studies will investigate the value of lipocalin-2 in respect of plaque progression in this study population.
Cardiac Computed Tomography
Leader: Univ.-Prof. Dr. Guy Friedrich
Members: Univ.-Prof.in Dr.in Gudrun Feuchtner; Dr. Fabian Plank
Based on the invasive and non-invasive imaging of coronary artery and structural heart disease, we have promoted international cooperation and executed self-initiated projects.
Post-procedural imaging of prosthetic heart valve dysfunction is a major challenge in cardiology. In cooperation with St. Paul’s Hospital in Vancouver and the Medical University of South Carolina, we found the optimal application of echocardiography and computed tomography to identify underlying pathology.
Coronary artery disease has evolved tremendously through plaque characterisation in non-invasive imaging. With two major manuscripts, cross-sectional and longitudinal analysis of diseased segments has aided understanding of the process of coronary atherosclerosis.
This has further enhanced the identification of vulnerable plaques and the prediction of major adverse cardiac events.
Fig. 3: Precise planning prior to invasive treatment
A major contribution towards improved patient care can be found in a multicentre trial sponsored by the European Union. With prospective patient randomisation between invasive angiography and cardiac computed tomography, an increase in diagnostic yield and a reduction in unnecessary invasive diagnostics are expected in the coming publication of primary endpoints.
Internal cooperation has further enhanced cardiac comorbidities, e.g. shared clinical presentation of patients with COPD and coronary artery disease. Together with our pulmonologists, we have shown delayed coronary evaluation in patients with COPD in comparison with patients without lung disease.
Experimental Electrophysiology
Leader: Univ.-Doz. DDr. Wolfgang Dichtl
Members: Univ.-Doz. Dr. Markus Stühlinger; Univ.-Doz. Dr. Wilfried Schgör; Univ.-Doz. Dr. Florian Hintringer; Dr. Fabian Barbieri; Dr. Thomas Senoner; Dr.in Agne Adukauskaite; Dr.in Andrea Rubatscher
The team focuses on clinical research into atrial fibrillation (rhythm control and thromboembolic prophylaxis), device therapy in heart failure (optimal use of ICD and CRT) and rare diseases (WPW syndrome, pacing-induced cardiomyopathy, Takotsubo syndrome, spontaneous coronary artery dissection and Friedreich ataxia). There is increasing interest in the use of deep learning and non-invasive imaging methods for risk stratification, in the case of arrhythmias, aortic stenosis or asymptomatic coronary artery disease.
Selected Publications
- Bauer A, Klemm M, Rizas KD, et al.: Prediction of mortality benefit based on periodic repolarisation dynamics in patients undergoing prophylactic implantation of a defibrillator: a prospective, controlled, multicentre cohort study. Lancet 2019;394:1344-51.
- Holzknecht M, Reindl M, Tiller C, Lechner I, Perez Cabrera R, Mayr A, Brenner C, Klug G, Bauer A, Metzler B, Reinstadler SJ (2020): Clinical Risk Score to Predict Early Left Ventricular Thrombus After ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Imaging. 2020; Epub ahead of print.
- Reindl M, Tiller C, Holzknecht M, Lechner I, Beck A, Plappert D, Gorzala M, Pamminger M, Mayr A, Klug G, Bauer A, Metzler B, Reinstadler SJ (2019): Prognostic Implications of Global Longitudinal Strain by Feature-Tracking Cardiac Magnetic Resonance in ST-Elevation Myocardial Infarction. Circ Cardiovasc Imaging. 2019;12(11):e009404.
- Reindl M, Tiller C, Holzknecht M, Lechner I, Hein N, Pamminger M, Henninger B, Mayr A, Feistritzer HJ, Klug G, Bauer A, Metzler B, Reinstadler SJ (2020): Aortic Stiffness and Infarct Healing in Survivors of Acute ST-Segment-Elevation Myocardial Infarction. J Am Heart Assoc. 2020;9(3):e014740.
- Reinstadler SJ, Stiermaier T, Reindl M, Feistritzer HJ, Fuernau G, Eitel C, Desch S, Klug G, Thiele H, Metzler B, Eitel I: Intramyocardial haemorrhage and prognosis after ST-elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2019;1;20(2):138-146.
- Messner M, Ghadge SK, Maurer T, Staggl S, Maier SC, Pölzl G, Zaruba MM.: ZMPSTE24 Is Associated with Elevated Inflammation and Progering mRNA. Cells. 2020 Aug 28;9(9): E1981. doi: 10.3390/cells9091981.
- Messner M, Ghadge SK, Schuetz T, Seiringer H, Pölzl G, Zaruba MM.: High Body Mass Index is Associated with Elevated Blood Levels of Progerin mRNA. INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES. 2019. 20(8)
- Beyer C, Wildauer M, Feuchtner G, Friedrich G, Hintringer F, Stühlinger M, Plank F.: JACC Cardiovasc Imaging. 2020;13:169-170.
- Kocher F, Kaser A, Escher F, Doerler J, Zaruba MM, Messner M, Mussner-Seeber C, Mayr A, Ulmer H, Schneiderbauer-Porod S, Ebner C, Poelzl G.: Heart failure from ATTRwt amyloid cardiomyopathy is associated with poor prognosis. ESC Heart Fail. 2020 Oct 1. Online ahead of print
- Escher F, Senoner M, Doerler J, Zaruba MM, Messner M, Mussner-Seeber C, Ebert M, Ensinger C, Mair A, Kroiss A, Ulmer H, Schneiderbauer-Porod S, Ebner C, Poelzl G.: When and how do patients with cardiac amyloidosis die? Clin Res Cardiol. 2020 Jan;109(1):78-88.
- Adukauskaite A, Barbieri F, Senoner T, Plank F, Knoflach M, Boehme C, Hintringer F, Müller S, Cartes-Zumelzu F, Dichtl W, Feuchtner G. (2019): Left atrial appendage morphology and left atrial wall thickness predict embolic cryptogenic stroke: a cardiac CT angiography study. JACC Cardiovasc Imag. 12: 2079-2081
- Plank F, Stowasser B, Till D, Schgör W, Dichtl W, Hintringer F, Weiss G, Stühlinger M. (2019): Reduction of fluoroscopy dose for cardiac electrophysiology procedures: A feasibility and safety study. Eur J Radiol. 110: 105-111
- Dichtl W, Tuovinen N, Barbieri F, Adukauskaite A, Senoner T, Rubatscher A, Hintringer F, Siedentopf C, Bauer A, Gizewski ER, Steiger R. (2020): Functional neuroimaging in the acute phase of takotsubo syndrome: volumetric and functional changes in the right insular cortex. Clin Res Cardiol. 109: 1107-1113
Selection of Funding
- Univ.-Prof. Dr. Axel Bauer, Austrian Science Fund (FWF): KLI-900
- Univ.-Prof. Dr. Axel Bauer, Deutsches Zentrum für Herzkreislaufforschung: SMART-MI Early Clinical Trial
- Univ.-Prof. Dr. Axel Bauer, European Commission: FP7-HEALTH-2013-INNOVATION-1 (WP1)
- Univ.-Prof. Dr. Bernhard Metzler, FWF KliF 772: „Soluble Neprilysin in ST-elevation Myocardial Infarction“
- Dr. Martin Reindl, PhD: Presidential Scholarship of the “Austrian Society of Cardiology”
- Priv.-Doz. Dr. Sebastian Reinstadler, PhD: Research Grant of the Austrian Heart Fund
- Dr. Martin Reindl, PhD: TWF-Grant: “Plasma proprotein convertase subtilisin kexin type 9 (PCSK9) in ST-elevation myocardial infarction: The PCSK9-STEMI trial”
- Univ.-Prof. Dr. Marc Zaruba, FWF P28817-B28: "Smooth muscle specific role of SDF-1 in cell recruitment and cardiac repair after MI”
- Univ.-Prof. Gerhard Pölzl: Research funding Tyrol, Identifizierung passgenauer Therapien gegen COVID-19: Telegesundheitssystem für COVID-19 Hochrisikopatienten
Collaborations
- German Center for Cardiovascular Research (DZHK), Germany
- Prof. Dr. G. Schmidt, Technische Universität München, München, Germany
- Prof. Dr. M. Zabel, Universitätsmedizin Göttingen, Göttingen , Germany
- Prof. Marek Malik, Cardiac and Vascular Sciences, St. George's, University of London, London, United Kingdom
- Prof. Wojciech Zareba, Heart Research Follow-up Program, University of Rochester, Rochester , USA
- Prof. Dr. H. Huikuri, Tampere University Hospital, Tampere, Finland
- Prof. Dr. H. Thiele, University of Leipzig, Leipzig, Germany
- Prof. Dr. I. Eitel, University of Lübeck, Lübeck , Germany
- Prof. Dr. S. Greulich, University Hospital Tübingen, Tübingen ,Germany
 Univ.-Prof. Dr.med. Axel Bauer
Univ.-Prof. Dr.med. Axel Bauer
Director
Contact:
Anichstraße 35
6020 Innsbruck
Austria
Email: Axel.Bauer@i-med.ac.at
Phone: +43 512 504 25621
Fax: +43 512 504 25622
http://inneremed3.tirol-kliniken.at



