Neurobiochemistry
Research Focus
Research
Selected Publications
Selected Funding, Collaboration
Devices & Services
Keywords: Nogo receptors, axon guidance, development, stroke models, circuits, Neuroscience, Biochemistry, Neurobiology, Molecular Biology, Cell Biology, Genetics, neuronal regeneration
Research (ÖSTAT Classification) : 1106002, 106010, 106013, 106023, 106052, 301402
Research Focus
The Institute is home to three independent research groups, whose scientific interests range from investigating molecular, cellular and biochemical aspects of axon development and regeneration to studying basic processes or potential therapeutic interventions of ischemic stroke. Since December 2020, the institute is also host of a new group focusing on translational models of psychiatric diseases and cognitive processes. Moreover, the Institute runs a state-of-the art bioimaging core facility which provides access to instruments, technologies and services to researchers of the MUI and other research institutions.
The aim of all research groups is to transfer promising basic research results into practice. Research activities at the Institute are supported by continuous intra and extra mural funds (FWF), with some members of the Institute participating in network programs, such as the FWF-funded PhD program “Signal Processing in Neurons”. Academic staff members at the Institute of Neurobiochemistry are also highly engaged in teaching of human medicine, dental medicine, molecular medicine (bachelors’ and masters’) and PhD curricula. Christine Bandtlow also began her tenure as Vice President for Research and International Relations in 2013 and was reelected by the University Council in 2017 and 2020.
Research
Molecular Basis of Axon Guidance and Target Selection
Christine Bandtlow, Maja Klose, Giuseppe Vaccaro, Dominik Brück, Florian Hofer
Research in the Bandtlow lab is focused on understanding how axonal guidance is orchestrated at the molecular level. The approach is based on combining molecular biology, cell biology, imaging, and genetics to define the role of molecules in both orchestrating axon growth during development and stabilizing neuronal circuits during adult life. The lab studies primarily the role of Nogo receptors in the peripheral sensory nerves of mouse mutants as a model, but also perform some studies on chicken commissural spinal cord. Current projects in the lab include:
1) Nogo Receptors (NgRs) as Guidance Molecules in the Developing Spinal Cord
We and others have shown that Nogo receptors (NgRs), particularly NgR1, play important roles in axonal regeneration and synaptic plasticity of the injured adult central nervous system. We now addressed the question whether NgRs play a physiological role in the developing nervous system. In collaboration with Esther Stöckli´s lab in Zurich, we were able to provide evidence that indeed NgR1 and NgR3 are required for commissural axon pathfinding in the chicken spinal cord in vivo. Furthermore, this led us to discover a functional cooperation between NgR1 and the axon repulsion receptor PlexinA2 during neurodevelopment. Overall, these findings provide a link between neural regenerative mechanisms with axon guidance developmental processes.
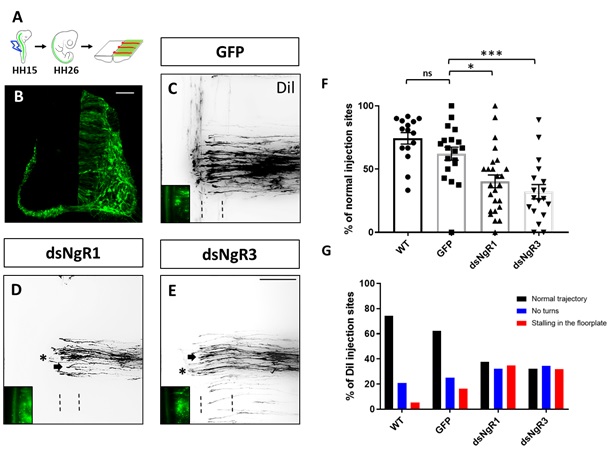
Fig. 1: Loss of either NgR1 or NgR3 interferes with dI1 axon guidance. (A) Representation of an open-book preparation from a HH26 chicken embryo spinal cord injected with the axonal tracer Dil (Andermatt et al., 2014). The embryos were injected with either a GFP expression plasmid alone or in combination with dsRNA. (B) Transverse spinal cord section of a chicken embryo electroporated unilaterally with a GFP plasmid. (C, D, E) Images of Dil traced commissural axons at the floorplate (indicated by dashed lines) and co-electroporated GFP-expressing neurons (bottom left) from embryos injected with the GFP plasmid alone (C) or together with either dsNgR1 (D) or dsNgR3 (E) (arrows indicate neurons stalling in the floorplate and asterisks indicate neurons not able to turn after crossing the midline). (F) Quantification of Dil injection sites with normal trajectories and (G) quantification of injection sites with axons turning normally, not turning and stalling in the floorplate in untreated chicken embryos (WT) or in control treated embryos injected with GFP alone or experimental embryos after electroporation with dsNgR1 or dsNgR3. ***P<0.001, *P<0,05, one-way ANOVA followed by Tukey’s test; error bars indicate s.e.m. Scale bar 50 µm in B and 100 µm E.
2) The Role of Nogo Receptors for Target Innervation in the Tongue
Taste buds are innervated by gustatory neurons of the geniculate ganglion via the chorda tympani nerve. Although taste placodes develop before sensory innervation, maturation and maintenance of taste buds largely depend on appropriate fiber innervation. Here we could show that Nogo receptors are dispensable for axon guidance, but are required for proper target innervation of taste placodes and thus for taste bud maturation.
Neuronal Checkpoints in Brain Development and Stroke Pathology
Gabriele Baier-Bitterlich, Dido Obexer (50%), Stephanie zur Nedden, Motahareh Solina Safari
A central focus of our lab is to analyze the function of the serine/threonine kinase Protein kinase N1 (PKN1) in the central nervous system. Our research focuses on the development of the cerebellum, hippocampus and retina, all of which are areas with abundant PKN1 expression. We further study the pathophysiological role of PKN1 in acute ischemia-reperfusion injury. Our research projects are supported by a murine PKN1 knockout model, cellular, biochemical as well as histological staining techniques.
1) Role of PKN1 in brain development
We have recently shown that PKN1 is a developmentally active regulator of cerebellar synaptic maturation by inhibiting AKT and the neurogenic transcription factor neurogenic differentiation factor-2 (NeuroD2) (zur Nedden et al, JCI, 2018). PKN1 deletion results in hyperactivated AKT and increased NeuroD2 expression, thereby causing enhanced axonal outgrowth and presynaptic spacing of cerebellar granule cells. We could reveal a similar effect of PKN1 deletion on AKT and NeuroD2 in the developing hippocampus, where it was accompanied by an increased expression of the NeuroD2 downstream target AMPAR subunit GluA1 (Safari et al, 2021). Our data suggest that the tight control of postnatal AKT/NeuroD2 levels by PKN1 constitutes a general and important regulatory mechanism in the development of several brain areas and therefore validates PKN1 as a novel keyplayer in neurodevelopment and neurodevelopmental disorders.
2) Preclinical evaluation of PKN1 as novel therapeutic target for ischemic stroke
Since the activation of the phosphoinositide-3-kinase/AKT cell survival-signaling pathway has become a promising new target for neuroprotective interventions, we are also studying the role of PKN1 during ischemia/reperfusion injury. We are employing various in vitro, ex vivo and in vivo models to analyze the role of PKN1 in pathophysiological conditions.
3) Role of PKN1 in retinal development and degeneration (Stephanie zur Nedden)
The main cause of incurable loss of vision lies in a permanent loss of retinal ganglion cells, the cells that give rise to the optic nerve and link the eye to the brain. A complete understanding of the developmental processes regulating the correct eye-brain connection is fundamental for the development of therapies that aim to restore vision. In the retina both, NeuroD2 overexpression and NeuroD2 knockout, affect retinal cell type formation and stratification, showing the importance of a tightly controlled balance of NeuroD2 protein levels for retinal development. This project will be dedicated to the analysis of the novel PKN1-AKT-NeuroD2 axis in the development of the visual pathway and its role in acute cellular stress-induced loss of retinal ganglion cells.
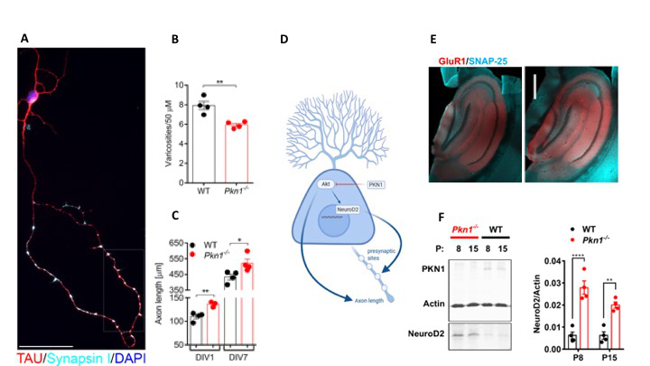
Fig. 2: PKN1 functions as a developmentally active gate keeper of AKT and NeuroD2
(A) In WT Cerebellar granule cells (Cgc) TAU-stained axonal en passant swellings colocalized with the presynaptic marker Synapsin I (images were taken at DIV4 and are representative of at least 3 separate experiments in WT and Pkn1-/- Cgc). (B) The number of en passant swellings (varicosities) per axonal section was analyzed in WT and Pkn1-/- Cgc at DIV7 (two-tailed unpaired t-test, t(6) = 4.413, (**) P = 0.0045, n = 4 WT/4 Pkn1-/- Cgc preparations from 4 litters/group (C) Axonal length after DIV1 (two-tailed unpaired t-test, t(5) = 4.431, (**) P = 0.0068, n = 4 WT/3 Pkn1-/- Cgc preparations from 3-4 litters/group) and DIV7 (two-tailed unpaired t-test, t(6) = 2.692, (*) P = 0.0360, n = 4 WT/4 Pkn1-/- Cgc preparations from 4 litters/group) in TAU-stained Cgc. (D) PKN1 is as a developmentally active enzyme regulating axon growth, presynaptic maturation, and synapse formation in the Parallel fiber (PF)-forming Cgc (zur Nedden et al, JCI, 2018). PKN1 deletion results in hyperactivated AKT and increased NeuroD2 expression, thereby causing enhanced axonal outgrowth and presynaptic spacing of Cgc. We could reveal a similar effect of PKN1 deletion on AKT and NeuroD2 in the developing hippocampus, where it was accompanied by an increased expression of the NeuroD2 downstream target AMPAR subunit GluA1. (E) (B) Hippocampal GluA1 levels were assessed by immunofluorescence staining. Pictures are representative of 3 animals/genotype. Scale bar in overview images refers to 500 mm and scale bar in high resolution inserts refers to 20 mm. (F) NeuroD2 expression in hippocampal whole cell protein extracts of P8 and P15 old WT and Pkn1-/- animals was assessed by western blotting (two way ANOVA: Interaction: P = 0.0745, Age: P = 0.0747, Genotype: P < 0.0001, Sidak’s multiple comparisons test: P8: P < 0.0001, P15: P = 0.0025) (Safari et al, frontiers, 2021).
Awards:
Prof. Brandl-Preis (Stephanie zur Nedden) October 2020
Schweigreiter Lab
Research in the Schweigreiter lab is focused on neuronal regeneration. Specifically, we aim to identify molecular mechanisms that govern and restrict the capacity for neuronal regeneration in the adult mammalian organism. On the one hand, we investigate axonal regeneration in the peripheral nervous system by making use of a newly developed method to visualize regenerating nerve fibres within nerve conduits. On the other hand, we recently became interested in novel transcription factor-mediated mechanisms that protect neurons of the central nervous system against various forms of stress. We currently explore the potential of this finding for stabilizing dopaminergic neurons during early stages of Parkinson's disease.
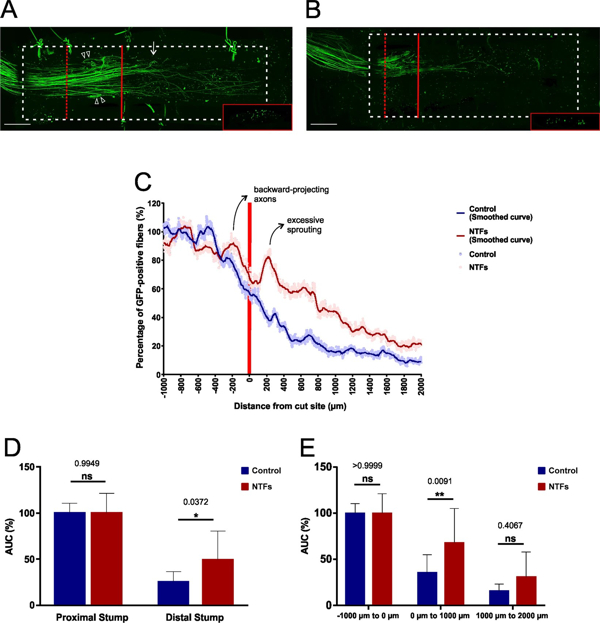
Fig. 3: Documenting the growth-promoting effect of neurotrophic factors during early-stage axonal regeneration within conduits. (A) Following sciatic nerve transections pre-treated chitosan conduits filled with PuraMatrix that had been supplemented with a cocktail of NTFs (final concentration: 2 µg/ml each of NGF, BDNF, NT-3, and GDNF) were implanted in the animals. After a regeneration period of 14 days the lesioned nerve was dissected and the nerve-conduit assembly then fixed, cleared and imaged by long working distance confocal microscopy. Excessive axonal sprouting immediately distal to the lesion site is indicated with a white arrow, backward-projecting axons within the proximal stump are marked with white arrow heads. The lesion plane is indicated with a red bar, the (y, z) section plane with a dashed red bar (see insert). The conduit’s silhouette is marked with a dashed white line. Scale bar length is 500 µm. (B) In the control group no NTFs were added to the PuraMatrix. (C) Quantification of the regenerative axonal growth at the lesion site, in the presence of NTFs and in their absence. Excessive axonal sprouting and the backward-projecting axons are marked on the NTF treatment curve. The curves from 10 independent experiments were pooled in each group. Data are presented as mean values at each incremental position. Solid lines have been smoothed over 50 µm intervals. (D) Statistical analyses were performed for the proximal and the distal segments, as well as for each of the three 1000 µm segments. n = 10 for each experimental group. Data represent mean ± SD. p values are indicated. *p ≤ 0.05, **p ≤ 0.01 (from Fogli B et al. 2019).
Neuro Alpine Lab
Johannes Passecker
The lab of Johannes Passecker is a very recent addition to the Institute focusing on how neuronal networks support cognitive control in both health and disease. Long-term or drastic misinterpretation of decision outcomes can lead to a range of behavioral and neurophysiological coping mechanisms often resulting in psychiatric conditions and symptoms. At the same time, maladaptive developmental processes can lead to similarly detrimental outcomes with measurable neuropathological manifestations. Our aim is to improve the understanding neurobiological foundation of these processes and develop better model systems for stringent and efficient testing of potential rescue options of psychiatric disorders. We pursue an integrated approach by applying novel and state-of-the-art techniques that include Head-fixed and freely moving in-vivo Electrophysiology, Optogenetics, Genetic Mouse Lines, RNA-analysis, Immunohistochemistry, Behavioural testing, Machine Learning and Deep-learning assisted Analysis.
The current project “Sexual dimorphism in GSK3 mediated rescue of working memory deficit“ is supported by the Behaviour and Brain Foundation in collaboration with the Integrative Neuroscience Section of the National Institute of Neurological Disorders and Stroke as well as the Joseph Gogos Lab at Columbia University.
Selected Publications
- The Prenylflavonoid ENDF1 Overrules Central Nervous System Growth Inhibitors and Facilitates Regeneration of DRG Neurons. Bieler L, Vogl M, Kirchinger M, Urmann C, Riepl H, Bandtlow C, Klimaschewski L, Aigner L, Couillard-Despres S. Front Cell Neurosci. 2019 Jul 24;13:332. doi: 10.3389/fncel.2019.00332. eCollection 2019.
- Dassati S*, Schweigreiter R*, Buechner S, and Waldner A (2021) Celecoxib promotes survival and upregulates the expression of neuroprotective marker genes in two different in vitro models of Parkinson's disease. Neuropharmacology 194:108378. doi: 10.1016/j.neuropharm.2020.108378 *...corresponding authors
- Schweigreiter R, Cawthorne C, Lippens S, Van Minnebruggen G, and Munck S (2020) Collaborating by courier, imaging by mail. EMBO Rep. 21: e49755. doi: 10.15252/embr.201949755
- Lamberti G, De Smet CH, Angelova M, Kremser L, Taub N, Herrmann C, Hess MW, Rainer J, Tancevski I, Schweigreiter R, Kofler R, Schmiedinger T, Vietor I, Trajanoski Z, Ejsing CS, Lindner HH, Huber LA, Stasyk T (2020) LAMTOR/Ragulator regulates lipid metabolism in macrophages and foam cell differentiation. FEBS Lett. 594: 31-42. doi: 10.1002/1873-3468.13579
- Fogli B, Corthout N, Kerstens A, Bosse F, Klimaschewski L, Munck S, Schweigreiter R (2019) Imaging axon regeneration within synthetic nerve conduits. Sci Rep. 9: 10095. doi: 10.1038/s41598-019-46579-w
- Anner P., Passecker J., Klausberger T., Dorffner G., Ca2+ imaging of neurons in freely moving rats with automatic post hoc histological identification. J. Neuroscience Methods, 06/2020 DOI: https://doi.org/10.1016/j.jneumeth.2020.108765
- Padilla-Coreano N., Canetta S., Mikofsky RM., Always E., Passecker J., Myroshnychenko M.V., Garcia-Garcia A.L., Warren R., Teboul E., Blackman D.R., Morton M. P., Hupalo S., Tye K. M., Kellendonk C., Kupferschmidt D. A., Gordon J. A., Hippocampal-prefrontal theta transmission regulates avoidance behavior, Neuron, Nov. 06/2019, DOI: 10.1016/j.neuron.2019.08.006
- Schwartenbeck P., Passecker J., Hauser T., FitzGerald T., Kronbichler M., Friston KJ. (2018) Computational mechanisms of curiosity and goal-directed exploration Elife. 2019 May 10;8. pii: e41703. doi: 10.7554/eLife.41703.
- Passecker J., Mikus N., Malagon-Vina H., Anner P., Dimidschtein J., Fishell G., Dorffner G., Klausberger T., Activity of prefrontal neurons predict future choices during gambling. Neuron. 2019 Jan 2;101(1):152-164.e7, DOI: 10.1016/j.neuron.2018.10.050.
Selection of Funding
- FWF, W1206: Doctoral College ́Signal processing in neurons ́
- Schweigreiter R (2020) Improving axon regeneration in the peripheral nervous system. FWF P 33411-B
- NARSAD Young Investigator Award 2019, Behaviour & Brain Foundation 2020-2022 (Passecker)
- FWF project P31085 (Gabriele Baier-Bitterlich)
- FWF Hertha Firnberg Fellowship T1091 (Stephanie zur Nedden)
Collaborations
- Esther Stöckli, University of Zurich, Department of Molecular Life Sciences and Neuroscience Center Zurich, Switzerland.
- Linda Barlow, University of Colorado, Anschutz Medical Campus, USA
- Sebastian Munck, VIB-KU Leuven Center for Brain & Disease Research, Belgium
- Sarah Dassati and Andreas Waldner, Melittaklinik S.R.L., Bolzano, Italy
Devices & Services
Core Facility BioOptics:
Microscopes: SP5, Neurolucida, iMIC, SP8 gSTD microscope, DMi8 inverted widefield microscope, LSM980 AiryScan, 2Photon SP8
 Univ.-Prof. Dr. Christine Bandtlow
Univ.-Prof. Dr. Christine Bandtlow
Director
Contact:
Biocenter, Innrain 80-82
6020 Innsbruck
Austria
Email: Christine.Bandtlow@i-med.ac.at
Phone: +43 512 9003 70281
Fax: +43 512 9003 73110
https://www.i-med.ac.at/neurobiochemistry/



