Physiology
Research Focus
General Facts
Research
Selected Publications
Selected Funding, Collaboration
Keywords: Voltage-gated calcium channels, nociception, iPSC derived human model systems, epithelial physiology
Research (ÖSTAT Classification) : 301407, 106006
Research Focus
Scientific research at the Institute of Physiology focuses on basic and preclinical translational experimental work in the areas of neurophysiology, the physiology of muscle and epithelial organs and recently the development of human model systems derived from human iPSCs (induced pluripotent stem cells). Current research projects include the science of normal biomolecular functions of humans in good health, their organs and the cells of which they are composed. Understanding of healthy bodily functions lays the foundations for exploring the pathogenesis of common diseases, such as renal dysfunction, chronic pain and many others. Our research is based on cellular models, organs and systems, and it employs an integrated, interdisciplinary approach. Particular innovation potential arises from ongoing projects exploiting human model systems and microRNAs as novel biomarkers and druggable targets for chronic neuropathic pain disorders.
General Facts
The institute is heavily involved in medical research and in teaching human physiology to undergraduate as well as graduate students at all levels. Understanding the healthy function of organisms and their parts is key to understanding processes that occur in diseased states. The institute’s research groups are involved in cutting-edge research on nociception, auditory function, calcium signalling, cell membranes and epithelial physiology. We employ a wide range of models and techniques including cell culture, functional imaging, gene and protein expression, calcium microfluorimetry, high-resolution live microscopy and electrophysiology, and we team up with local, national and international consortia. We are grateful for funding provided by the European Commission, the FWF, the FFG and others. The FWF-Docfunds project CavX is chaired by G. Obermair, M. Kress is a beneficiary of MSCA-ITN TOBeATPAIN funded by the European Commission, and two-photon microscopy is provided as a service of the institute.
Research
Neurobiology of Pain
Univ.-Prof. Dr. Michaela Kress
Chronic pain disorders affect about 20% of people and therefore represent a major burden on society as well as patients and their families worldwide. Deregulated molecular signalling along the entire pain pathway contributes to pain pathogenesis. The focus of the research is particularly on neuroimmune interactions involving cytokines, especially interleukin-6 together with its signal transducer gp130, and microRNA as important regulatory hubs as well as neuroplastic alterations of prefrontal circuits (Fig. 1). In order to tackle pain pathogenesis, an interdisciplinary methodological approach is used, exploiting cutting-edge electrophysiology, molecular biology together with complex bioinformatics, and human iPSC-derived model systems.
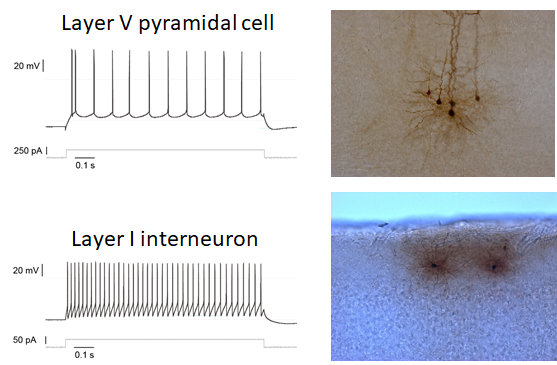
Fig. 1: PFC pyramidal cells and interneurons differ in firing pattern and morphology.
Calcium Channel Function
Voltage-gated calcium channels (VOCCs) convert electrical signals into important cell functions, such as the contraction of skeletal and heart muscle, secretion of hormones and neurotransmitters, and information processing in the nervous system. Conversely, defective VOCCs cause a range of neurological and psychiatric disorders. Three research teams are using advanced molecular electrophysiology and microscopy techniques to study VOCC in health and disease.
Univ.-Prof. Dr. Bernhard E. Flucher
The research group elucidates the biophysical mechanisms by which VOCCs sense changes in membrane potential and thus activate calcium currents and excitation-contraction coupling with distinct kinetics and voltage sensitivity. Structure-function approaches are developed and applied, in order to study the mechanisms by which mutations in VOCCs give rise to muscle and brain diseases.
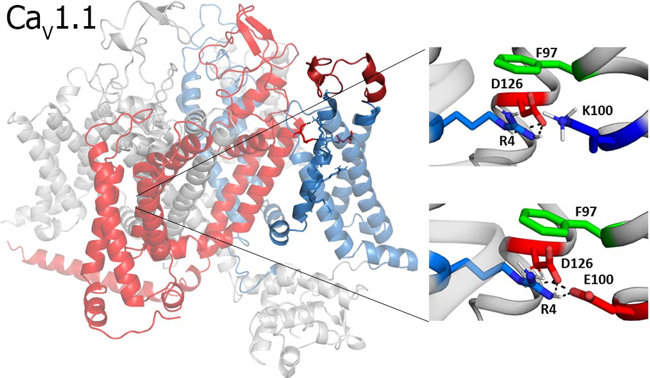
Fig. 2: Structure model of a voltage-gated calcium channel. The channel (left) consists of four voltage-sensing domains arranged symmetrically around a common pore. The close-ups (right) show how disease-causing mutations alter important functional interactions within a voltage-sensor.
Univ.-Prof. Dr. Gerald Obermair
VOCCs are also key regulators in the development and plasticity of synapses. The team studies how auxiliary alpha2delta calcium channel subunits determine the identity of excitatory and inhibitory synapses in the brain independently of channel function. G. Obermair also heads the FWF-funded PhD programme on Calcium Channels in Excitable Cells.
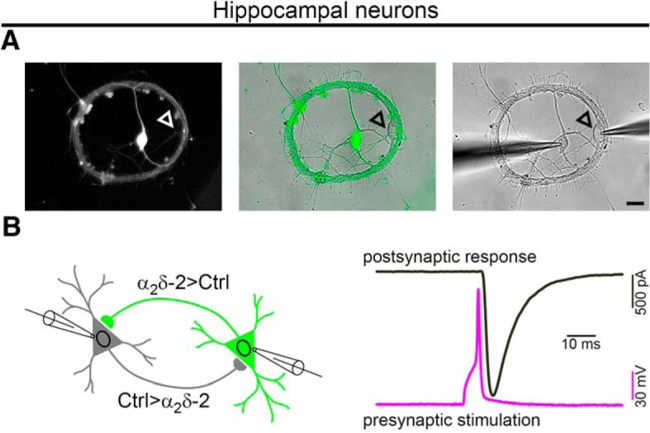
Fig. 3: Neuronal culture model to study the role of calcium channels in synaptic function. A. Miniature neuronal network between two neurons, allowing simultaneous electrophysiological recordings from presynaptic (green) and postsynaptic (arrowhead) neurons. B. Cartoon depicting the miniature network model (left) and an exemplary recording trace, showing presynaptic action potential (pink) and the postsynaptic response (grey).
Physiology of the Intestinal Barrier
ao. Univ.-Prof. Dr. Johannes Fürst
Epithelial barriers play an important role in the homeostasis of the body. The intestinal mucosa, for example, must provide a physical and chemical barrier to the environment and simultaneously allow the active and passive uptake of essential molecules such as nutrients. Various gastrointestinal diseases are associated with a dysregulated intestinal barrier. Our main interest lies in investigating the physiology of the intestinal barrier in vitro, with a focus on the role of neurotransmitters and cytokines on colonic enterocytes.
Respiratory Cell Physiology
ao. Univ.-Prof. Dr. Thomas Haller
Topics range from alveolar biology to interfacial phenomena, from surfactant exocytosis to drug sequestration in type-II cells and immunology. Although the main interest still lies in lung physiology, current research has begun on oxygen transport in red blood cells, with a special emphasis on haemoglobin in modulating oxygen transfer in the body. At present, 4 clinical trials have been completed, covering accidental hypoxaemia and the effects of inhaled vasodilator and volatile anaesthetics on HB-O2 affinity.
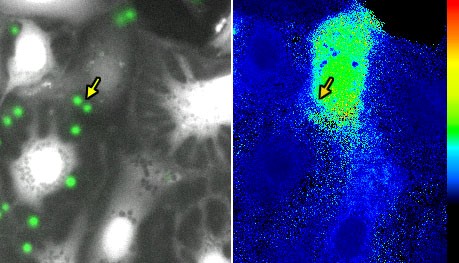
Fig. 4: GFP conidia (green, arrow) of A. fumigatus attached to alveolar type-II cells (left) and fura 2 Ca2+ signalling (right).
Human Kidney Model Systems
ao. Univ.-Prof. Dr. Judith Lechner
The research team uses iPSC lines obtained through the STEMBANCC initiative to establish and characterise novel, sex-specific, in vitro model systems by summarising sex differences in development through modification of iPSC-differentiation protocols. The differentiated cells are subjected to acute sex hormone treatments, in order to model the effects of the natural menstrual cycle or hormone changes due to menopause. These cells are used to study the mechanisms of sex differences in the cellular responses to environmental challenges and toxic stimuli.
Junior Research Groups
VOCC-Related Scaffolds
Dr. Marta Campiglio recently identified the molecular interactions between calcium channel and STAC3, a scaffold protein that is essential for skeletal muscle contraction. Her team is currently investigating the putative function of STAC3 as the missing link in skeletal excitation-contraction coupling.
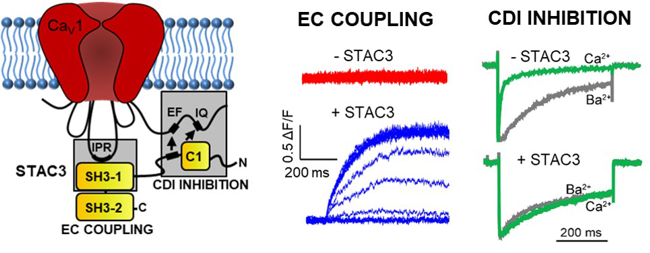
Fig. 5: CC BY 4.0: Costé de Bagneaux, Tuinte and Campiglio. STAC3 is essential for skeletal muscle function. Two interaction sites of STAC3 with CaV1 channels are important, respectively in order to mediate skeletal muscle contraction and to inhibit calcium-dependent inactivation.
Sound Encoding in Auditory Hair Cells
Priv.-Doz. Dr Michael Leitner aims to understand the molecular mechanisms of precise sound encoding in auditory inner/outer hair cells, a prerequisite for the enormous sensitivity and frequency discrimination of mammalian hearing (see Cell Rep. 2020 Jul 7; 32(1): 107869; J Neurosci. 2019 Nov 6; 39(45): 9013-9027).
Selected Publications
- Quarta S, Mitrić M, Kalpachidou T, Mair N, Schiefermeier-Mach N, Andratsch M, Qi Y, Langeslag M, Malsch P, Rose-John S, Kress M.: Impaired mechanical, heat, and cold nociception in a murine model of genetic TACE/ADAM17 knockdown. FASEB J.: 2019 33(3): S. 4418-4431.
- Campiglio, Marta, Costé de Bagneaux, Pierre, Ortner, Nadine, Tuluc, Petronel, Van Petegem, Filip, Flucher, Bernhard E.: STAC proteins associate to the IQ domain of CaV1.2 and inhibit calcium-dependent inactivation. PROC. NATL. ACAD. SCI. USA: 2018, 115. S. 1376-1381.
- Kaplan, Mehmet M., Sultana, Nasren, Benedetti, Ariane, Obermair, Gerald J., Linde, Nina F., Papadopoulos, Symeon, Dayal, Anamika, Grabner, Manfred, Flucher, Bernhard E.: Calcium influx and release cooperatively regulate AChR patterning and motor axon outgrowth during neuromuscular junction formation. CELL REPORTS: 2018; 23: S. 3891-3904.
- Geisler S, Schöpf CL, Stanika R, Kalb M, Campiglio M, Repetto D, Traxler L, Missler M, Obermair GJ.: Presynaptic α2δ-2 Calcium Channel Subunits Regulate Postsynaptic GABAA Receptor Abundance and Axonal Wiring. J NEUROSCI: 2019; 39:2581-2605.
- Schiefermeier-Mach, Natalia; Perkhofer, Susanne; Heinrich, Lea; Haller, Thomas: Stimulation of surfactant exocytosis in primary alveolar type II cells by A. fumigatus. MEDICAL MYCOLOGY. 2020; DOI: 10.1093/mmy/myaa042
- Dierich, Marlen; Altoè, Alessandro; Koppelmann, Julia; Evers, Saskia; Renigunta, Vijay; Schäfer, Martin K.; Naumann, Ronald; Verhulst, Sarah; Oliver, Dominik; Leitner, Michael G.: Optimized Tuning of Auditory Inner Hair Cells to Encode Complex Sound through Synergistic Activity of Six Independent K+ Current Entities. CELL REPORTS. 2020 Jul 7;32(1):107869
Selection of Funding
Stand alone projects:
- FWF, P28611: NIPPS (Kress)
- FWF, P30809: DynChomiR (Kummer)
- FWF, P30402: Regulation of voltage-sensitivity of CaV calcium channels (Flucher)
- FWF, P33270: The role of CaV1.1 calcium channels in presynaptic differentiation during neuromuscular junction development (Flucher)
- FWF, P33776, Analysis of the functions of multiple STAC interactions (Campiglio)
- TWF Tiroler Wissenschaftsförderung (MG Leitner)
DocFunds:
- FWF, DOC-30: PhD program: Calcium channels in excitable cells (Speaker: Obermair, PIs: Campiglio, Flucher)
Consortia:
- European Commission, MSCA-ITN “TOBeATPAIN” GA764860 (Kress, PI)
- FFG, Bridge1-Project “ceRNAPsych”, Projekt Nr. 853294 (Kress, Coordinator)
Translational projects:
Collaborations
- Filip Van Petegem, University of British Columbia, Canada
- Klaus Liedl, University of Innsbruck, Austria
- Jörg Striessnig, University of Innsbruck, Austria
- Markus Missler, Westfälische Wilhelms-University, Münster, Germany
- Tobias Moser, Institute for Auditory Neuroscience, University Medical Center Göttingen, Germany
- Markus Ritter, Paracelsus Medical University, Salzburg, Austria
- Hermann Brugger, Eurac Research, Bozen, Italy
- Hermona Soreq, Hebrew University Jerusalem, Israel
- Marzia Malcangio, King’s Collge London, United Kingdom
Devices & Services
- Two-Photon Microscopy (Core facility Biooptics)
 Univ.-Prof. Dr. med. Michaela Kress
Univ.-Prof. Dr. med. Michaela Kress
Contact:
Schöpfstr. 41
6020 Innsbruck
Austria
Physiologie@i-med.ac.at
Phone: +43 512 9003 70801
Fax: +43 512 9003 73800
www.i-med.ac.at/dpmp/physiologie



